The STTs comprise extraskeletal mesenchymal tissue tumours with heterogeneous histogenic lineages [1]. The diagnostic bases of STT rest upon the histologic diagnosis made by the routine light microscopic evaluation of Haematoxylin and Eosin (H&E) stained tissue sections with or without special stains. Yet, the diagnosis of tumours in limited biopsy samples is a challenge for surgical pathologists. Fortunately, there is a continuous identification of new adjunctive techniques like immunohistochemistry, electron microscopy, flow cytometry and cytogenetics. Such techniques which have increased our insight into the tumour pathogenesis and biology, act as tools for improved diagnostic accuracy. The newly developed immunomarkers have ushered in a contemporary diagnostic immunohistochemistry era as they have replaced the conventional lineage markers in some cases because they are much more specific and recognise proteins that are characteristic of molecular genetic alterations.
Materials and Methods
A cross-sectional analysis was done covering data of 11 years (January, 2009 to December, 2019). This study was conducted from January, 2018 to December, 2019. It enrolled 2683 cases of morphologically diagnosed STT; of these 1776 were retrospective and 907 were prospective. Cases were received in the Department of Histopathology in Central General and Vin Private Laboratories in Duhok-Iraq. The study received approval from the Duhok Directorate of Health with regard to the processing of personal data. Ethical approval to conduct the study was obtained from the Research Ethics Committee in Directorate of Health (numbered: 2422), and patient consent was obtained before enrolling participants in the study. Uterine leiomyomas (n=1797) were excluded and the remaining 886 STT were enrolled. Data were retrieved from the patients themselves and/or their reports. The operated tissue specimens were processed and stained with H&E and re-examined under light microscope to confirm the diagnosis. Cases were categorised morphologically according to the latest WHO recommendations [3].
Using monoclonal and polyclonal antibody panels, immunohistochemistry was performed on all query cases via the UltraVision LP Large Volume Detection System and HRP (horseradish peroxidase) Polymer (Ready-To-Use) from Thermo Fisher Scientific®. Antigen retrieval Heat Induced Epitope Retrieval (HIER) was performed with epitope retrieval solutions and the chromogen used was 3-3’-Diaminobenzidine Tetrahydrochloride (DAB). Detection kit was used according to the manufacturer’s recommendations and as described previously [4-13]. Representative tissue sections from formalin-fixed tumours were selected and embedded in paraffin blocks. Three μm unstained sections were taken and mounted on fisherbrand® positively charged glass slides, then placed in drying oven at 65°C for 12 hours, deparaffinised in xylene and then rehydrated in graded alcohol. Slides were mounted into autostainer racks and heated to the desired temperature according to the manufacturer’s recommendations and then cooled down to 60°C, followed by thorough washing with distilled water. These slides were rinsed thrice in Phosphate Buffered Saline (PBS, Dako Denmark A/S), incubated in Hydrogen Peroxide for 10 minutes, rinsed again in PBS, and then placed in Ultra V Block. After that, all sections were subjected to the first panel antibodies [Table/Fig-1] [4-13]. Then according to the results, second panel antibodies were added. Sections were counterstained with Mayer’s haematoxyllin, dehydrated through graded alcohols to xylene and then mounted with DPX (Distyrene Plasticizer Xylene) solution and cover slipped. Strongly positive controls and negative controls (using the same procedure without primary antibodies) were used with each run. The proliferative index (Ki-67) was used in suspected low-grade cancers [9-13].
Detailed antibodies used for malignant Soft Tissue Tumour (STT) categorisation [4-13].
| Marker | Task |
|---|
| First panel |
| Pan Keratin | For synovial sarcoma and to exclude carcinoma and carcinosarcoma |
| S-100 protein | For lipid and cartilaginous tumours and to exclude melanoma |
| Vimentin | For soft tissue/mesenchymal tumours |
| CD45 | Applied in round cell tumours to exclude lymphoma/leukaemia |
| Second panels |
| Sarcoma | Antibodies used |
| Synovial sarcoma | EMA, CD99, Bcl-2 |
| Rhabdomyosarcoma | Desmin, Myo D1, Myogenin |
| Myxofibrosarcoma | Desmin, SMA, CD99 |
| Low-grade fibromyxoid sarcoma | MUC4, CD99 |
| Myxofibrosarcoma | CD99 (not specific, used with others to exclude mimics) |
| DFSP | CD34 |
| Smooth muscle tumours | SMA, H-Caldesmon |
| Vascular tumours | CD31, CD34 |
| Ewing’s/PNET | CD99, Fli-1 in addition to PAS+/PASD-* |
| Liposarcoma | CDK4, S100 protein |
| MPNST | CD99, CD57 |
| Chondrosarcoma | CD99, Leu7, S100 protein |
| Kaposi sarcoma | D2-40, CD31, CD34, PAS+/PASD+ hyaline globules |
| Endometrial stromal sarcoma | CD10, ER |
DFSP: Dermatofibrosarcoma protuberans; MPNST: Malignant peripheral nerve sheath tumour; PNET: Primitive neuroectodermal tumour; *PAS/PASD: Periodic acid schiff/Periodic acid schiff/diastase special enzymes used in histochemistry, Bcl-2: B-cell lymphoma 2; CD: Cluster of differentiation; CDK4: Cyclin-dependent kinase 4; EMA: Epithelial membrane antigen; ER: Estrogen receptor; FLI1: Friend leukaemia integration 1; MUC: Mucin; SMA: Smooth muscle actin
Results
The study included 886 (768 benign and 118 malignant) STT. Patients’ age ranged from one month to 82 years (mean=38.4 years) with 1:1.4 male to female ratio. As shown in [Table/Fig-2], benign tumours dominated in the second- fifth decades of life, while malignant tumours dominated in the fourth, fifth decades and above 60 years. Both showed a peak in the fourth decade. The mean age of patients with benign tumours was 38.3 years while that of malignant tumours was 39.1 years.
Prevalence rates of STT among different age groups.
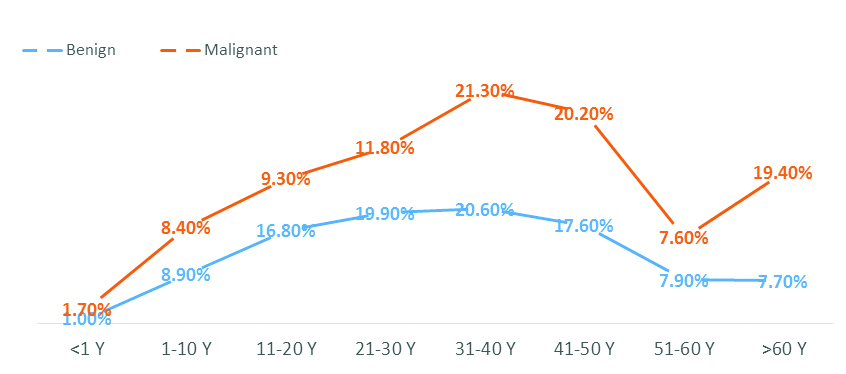
Soft Tissue Tumours (STT) and Location
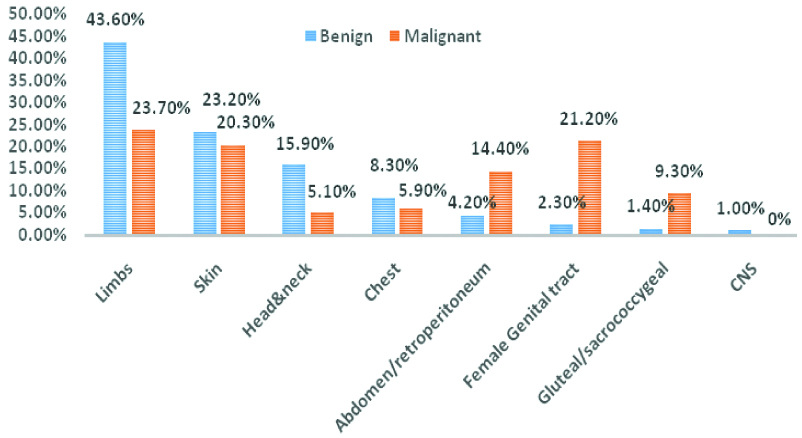
As presented in [Table/Fig-3], the most frequent location affected by both benign STT was the limbs (43.6%), followed by skin (23.2%) and head and neck (15.9%). The most common location for malignant tumour was also the limbs (23.7%) followed by female genital tract (21.2%) and skin (20.3%).
Soft Tissue Tumours (STT) and location.
Benign Soft Tissue Tumours (n=768)
As shown in [Table/Fig-4] (42.7%). Lipomas peaked in third-fifth decades of life; vascular tumours showed a peak in the first-fourth decades of life, while nerve sheath tumours, fibrohistiocytic neoplasms and fibromyofibroblastic tumours peaked in second-fifth decades of life. Neonatal cases comprised vascular and nerve sheath tumours. Chondro-osseous tumours were mainly observed in the second decade.
Benign Soft Tissue Tumours (STT) and age intervals.
| Tumour (T) Number (%) | Age groups (years) Number (%) |
|---|
| <1 | 1-10 | 11-20 | 21-30 | 31-40 | 41-50 | 51-60 | >60 |
|---|
| Adipocytic T: 328 (42.7) | 0 (0) | 15 (1.9) | 28 (3.9) | 61 (7.9) | 77 (10) | 80 (10.4) | 39 (5) | 28 (3.6) |
| Vascular T: 178 (23.2) | 5 (0.6) | 36 (4.6) | 44 (5.7) | 34 (4.8) | 30 (3.9) | 16 (2) | 5 (0.6) | 8 (1) |
| Nerve sheath T: 108 (14.1) | 3 (0.4) | 6 (0.8) | 24 (3.3) | 21 (2.7) | 20 (2.6) | 18 (2.3) | 11 (1.4) | 5 (0.6) |
| Fibrohistiocytic T: 95 (12.3) | 0 (0) | 5 (0.8) | 15 (1.9) | 25 (3.2) | 21 (2.7) | 15 (1.9) | 3 (0.4) | 11 (1.4) |
| Fibro/Myofibroblastic T: 47 (6.1) | 0 (0) | 6 (0.7) | 13 (1.7) | 10 (1.3) | 9 (1.2) | 3 (0.4) | 3 (0.4) | 3 (0.4) |
| Chondro-osseous T: 12 (1.6) | 0 (0) | 1 (0.1) | 5 (0.6) | 2 (0.4) | 1 (0.1) | 3 (0.4) | 0 (0) | 0 (0) |
| Total: 768 (100) | 8 (1) | 69 (8.9) | 129 (16.8) | 153 (19.9) | 158 (20.6) | 135 (17.6) | 61 (7.9) | 55 (7.2) |
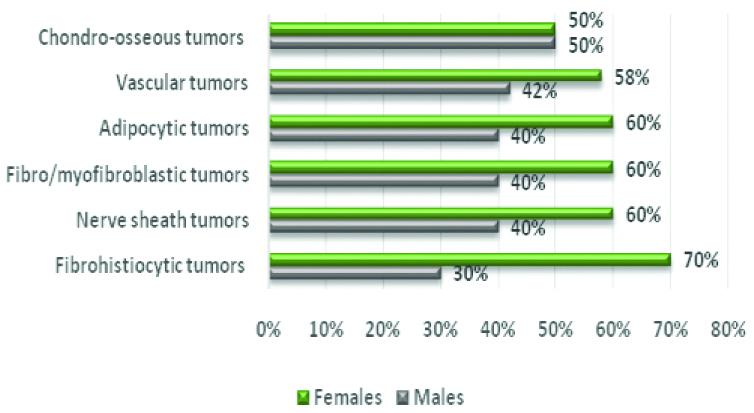
Concerning the sex distribution, benign tumours affected females (n=467; 60.8%) more than males (n=301; 39.2%). Individually, only chondro-osseous neoplasms showed equal sex distribution whereas all others showed a trend towards female predominance [Table/Fig-5]; however, the difference did not reach the level of significance (p<0.05).
Benign Soft Tissue Tumours (STT) and sex distribution.
Benign Soft Tissue Tumour Histologic Variants
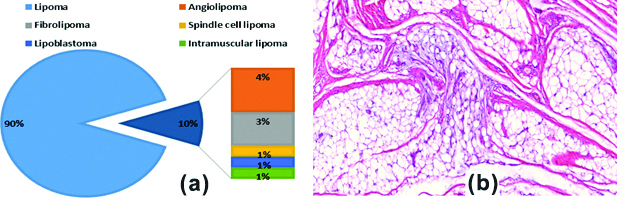
As illustrated in [Table/Fig-6a], conventional lipoma formed 90% of adipocytic tumours. The remainders comprised variable admixture of angiolipoma, fibrolipoma, spindle cell lipoma, intramuscular lipoma and lipoblastoma [Table/Fig-6b].
a) Percentages of benign adipocytic tumours; b) Lipoblastoma in a 12-year-old boy showing nests of mature fat globules with myxoid foci (H&E, X200).
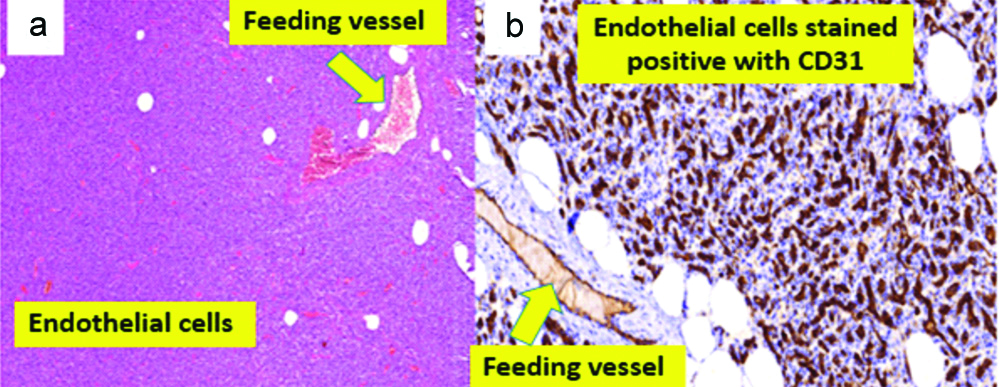
For statistical purposes, perivascular tumours were included alongside with the vascular tumours. They comprised capillary haemangioma in 66% [Table/Fig-7], lymphangioma (12.2%), cavernous haemangioma (12.2%) and glomus tumour (10%).
a) Highly cellular capillary haemangioma in an infant (H&E sections, X40). b) Positive for CD31 (IHC, X400).
Benign nerve sheath tumours included neurofibroma (45.4%), schwannoma (38%) [Table/Fig-8] neuroma (5.5%), ganglioneuroma (4.6%), and granular cell tumour (3.7%). Paraganglioma (2.8%) was included within nerve sheath tumours for statistical purposes.
a) Schwannoma: Antoni A showing typical palisading nerve fibers; b) Antoni B showing dystrophic calcification in a cystic/myxoid background (H&E, X100).
Malignant Soft Tissue Tumours
Sarcoma Not Otherwise Specified (NOS) formed the commonest malignant STT followed by leiomyosarcoma, rhabdomyosarcoma and Ewing’s/PNET, DFSP and liposarcoma, synovial sarcoma, MPNST and chondrosarcoma. Rare entities included Endometrial Stromal Sarcoma (ESS), Kaposi sarcoma and alveolar soft part sarcoma. Most sarcomas NOS were found among patients older than 60 years, whereas many others were demonstrated between third and fifth decades [Table/Fig-9].
Malignant Soft Tissue Tumours (STT) and age groups.
| Tumour (T) Number (%) | Age groups (years) Number (%) |
|---|
| <1 | 1-10 | 11-20 | 21-30 | 31-40 | 41-50 | 51-60 | >60 |
|---|
| Sarcoma, NOS: 29 (24.5) | 0 (0) | 1 (0.9) | 1 (0.9) | 3 (2.5) | 8 (6.8) | 4 (3.4) | 2 (1.7) | 8 (8.4) |
| Leiomyosarcoma: 24 (20.3) | 0 (0) | 0 (0) | 0 (0) | 3 (2.5) | 4 (3.4) | 8 (6.8) | 4 (3.4) | 5 (4.2) |
| RMS: 12 (10.1) | 0 (0) | 6 (5) | 4 (3.3) | 1 (0.9) | 0 (0) | 0 (0) | 0 (0) | 1 (0.9) |
| Ewing/PNET: 12 (10.1) | 2 (1.7) | 1 (0.9) | 6 (5) | 1 (0.9) | 2 (1.7) | 0 (0) | 0 (0) | 0 (0) |
| Liposarcoma: 11 (9.4) | 0 (0) | 0 (0) | 0 (0) | 1 (0.9) | 2 (1.7) | 5 (4.2) | 1 (0.9) | 2 (1.7) |
| DFSP: 11 (9.4) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 7 (6) | 3 (2.5) | 1 (0.9) | 0 (0) |
| Synovial sarcoma: 6 (5.1) | 0 (0) | 1 (0.9) | 0 (0) | 2 (1.7) | 2 (1.7) | 1 (0.9) | 0 (0) | 0 (0) |
| MPNST: 3 (2.5) | 0 (0) | 0 (0) | 0 (0) | 1 (0.9) | 0 (0) | 0 (0) | 0 (0) | 2 (1.7) |
| Chondrosarcoma: 3 (2.5) | 0 (0) | 0 (0) | 0 (0) | 1 (0.9) | 0 (0) | 0 (0) | 0 (0) | 2 (1.7) |
| Fibro/myofibroblastic tumours: 2 (1.7) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 2 (1.7) | 0 (0) | 2 (1.7)* |
| ESS: 2 (1.7) | 0 (0) | 0 (0) | 0 (0) | 1 (0.9) | 0 (0) | 1 (0.9) | 0 (0) | 0 (0) |
| Kaposi sarcoma: 2 (1.7) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (0.9) | 1 (0.9) |
| ASPS: 1 (0.9) | 0 (0) | 1 (0.9) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Total: 118 (100) | 2 (1.7) | 10 (8.7) | 11 (9.3) | 14 (11.8) | 25 (21.3) | 24 (20.2) | 9 (7.6) | 23 (19.4) |
NOS: Not otherwise specified, RMS: Rhabdomyosarcoma, PNET: Primitive Neuroectodermaltumour, DFSP: Dermatofibrosarcoma protuberans, MPNST: Malignant peripheral nerve sheath tumour, SS: Endometrial stromal sarcoma, ASPS: Alveolar soft part sarcoma; *The two cases of fibro/myofibroblastic tumours included a case of myxofibrosarcoma and a low-grade fibromyxoid sarcoma
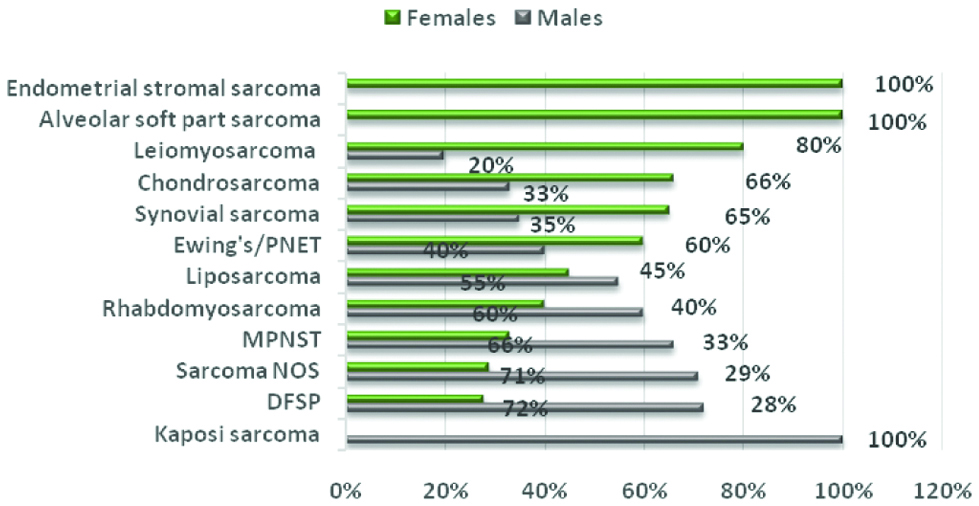
Considering sex distribution, ESS and alveolar soft part sarcoma were exclusively diagnosed in females, followed by (in decreasing female frequency) leiomyosarcoma (80%), chondrosarcoma (66%) and synovial sarcoma (65%). In contrast, Kaposi sarcoma was limited to males (100%), followed (with decreasing male frequency) by DFSP (72%), sarcoma NOS (71%), MPNST (66%), rhabdomyosarcoma (60%), Ewing’s/PNET (60%) and liposarcoma (55%) [Table/Fig-10].
Malignant soft tissue tumours and sex distribution.
DFSP: Dermatofibrosarcoma protuberans, NOS: Not otherwise specified, MPNST: Malignant peripheral nerve sheath tumour, PNET: Primitive Neuroectodermal tumour
Different morphological and immunohistochemical images of malignant STT are shown in [Table/Fig-11,12,13,14,15,16 and 17].
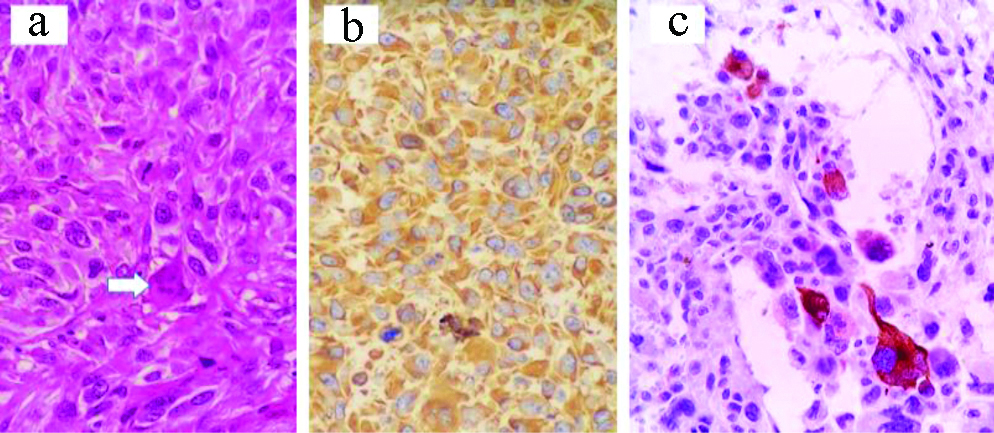
a) Sarcoma (NOS) with osteoclast-like multinucleated giant cells (white arrows) and focal muscle differentiation: H&E, X400; b) Vimentin IHC, X400; c) Desmin, X400.
a) Leiomyosarcoma: H&E, X400; b) IHC: H-Caldesmon, X400; c) IHC: High Ki67, X400.
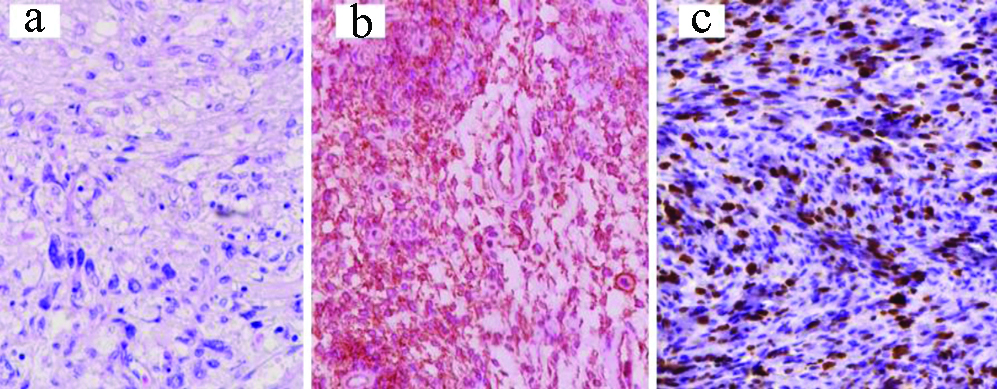
a) Liposarcoma: H&E, X100; b) CDK4 IHC, X400; c) Dedifferentiated area, S-100 protein IHC, X200.
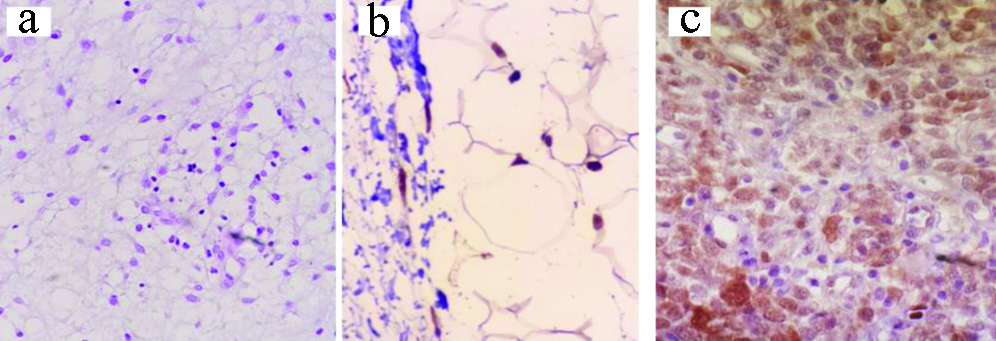
a) Rhabdomyosarcoma: H&E, X400; b) IHC: Desmin, X100; c) IHC: Myogenin, X400.
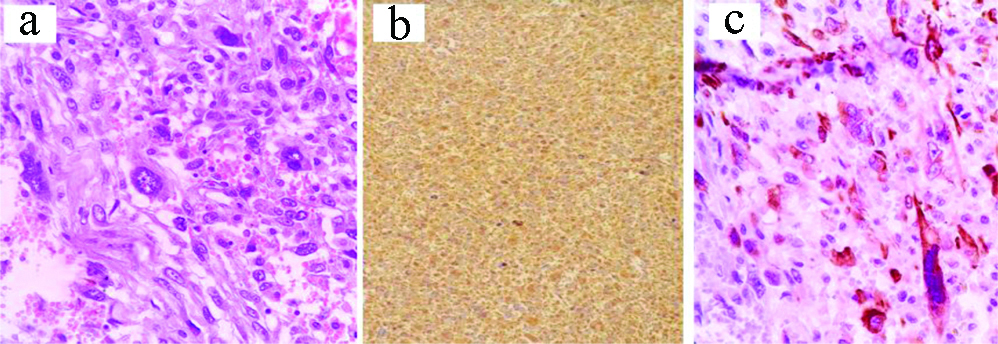
a) Dermatofibrosarcoma Protuberans (DFSP): H&E, X100; b) IHC: CD34, X100; c) IHC: CD34, X200.
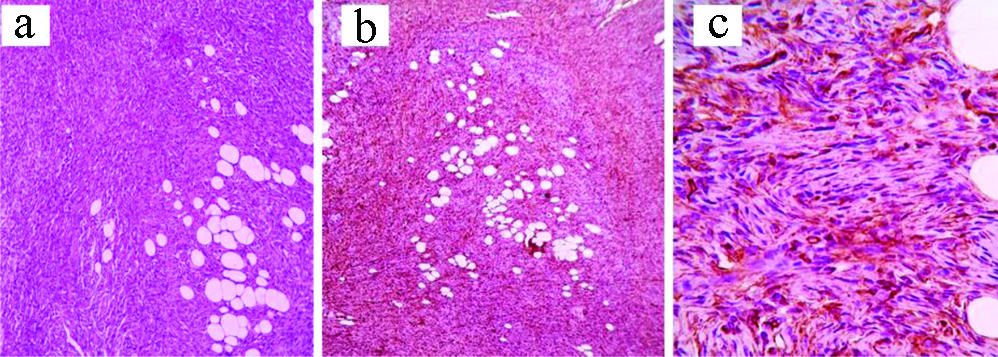
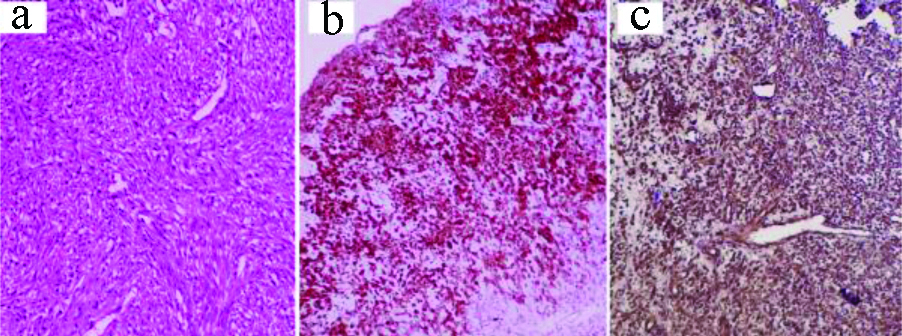
a) Myxofibrosarcoma: H&E, X100; b) H&E, X400; c) IHC: CD99, X400.
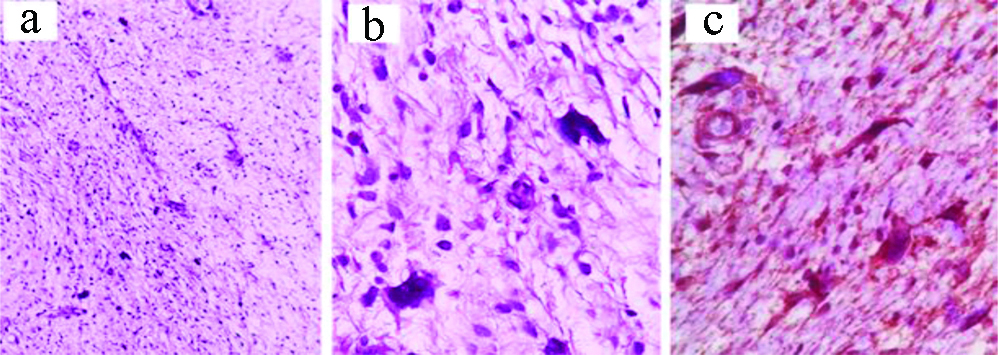
a) Synovial sarcoma: H&E, X200; b) IHC: Vimentin X100; c) IHC: CD99, X400).
Discussion
Benign Soft Tissue Tumours
In this study, benign STT overran malignant by a ratio of about 6.5:1. It has been reported that benign soft tissue neoplasms outnumber sarcomas by a factor of at least 100 [3]. Indeed, exclusion of 1797 uterine leiomyomas in this study may be one cause of lowering benign tumour rates because it is a common benign tumour in this locality [6].
Benign tumours dominated in the second-fifth decades of life and showed a peak in the fourth decade with no significant sex distribution. More or less similar findings have been reported by Jobanputra GP et al., [14].
It is worthy to state that neonatal benign tumours in this study were restricted to vascular and neuronal tumours (capillary haemangioma, lymphangioma, granular cell tumour and neurofibroma). In parallel, haemangiomas have been reported to be the most frequent STT seen in infants and paediatric age groups [1].
Adipocytic tumours topped the benign STT. This finding has been described by other authors as well [7,14,15]. Similarly, there was no gender predilection as reported by other authors including a study done on 93 STT [1,7,15,16].
Malignant Soft Tissue Tumours
Malignant STT were seen to be dominated at the fourth and fifth decades and above 60 years of age. No significant sex predilection was noted in accordance to studies performed in USA, India, France and Austria [14,17-19].
Regarding the location, these were most commonly diagnosed in the limbs, followed by female genital tract, skin and subcutaneous tissues. Limb predominance may be explained by the high frequency of liposarcomas in adult limbs and predominance of embryonal rhabdomyosarcoma and Ewing’s/PNET among youngest patients. This finding has been documented in other studies as well [20].
Immunohistochemically, all sarcomas showed vimentin positivity with variable density. However, even after application of different antibody panels, no specific categorisation tumours could be achieved because of lack of clear cut immunoreactivity in 24.5% tumours. This may be explained by loss of many differentiation antigens in high grade tumours [21]. These uncharacterised cancers were put under the name “sarcoma NOS” as described previously [4,22]. On the same lines, in a study done on 1558 sarcomas from three European regions, reported that 26% of malignant STT were pleomorphic undifferentiated sarcoma [23]. Yet higher frequency (36%) of sarcoma NOS was described in a retrospective Austrian study on 5333 registered sarcomas [19]. It is worth noting that a case of sarcoma NOS with focal muscle differentiation (focally Desmin positive) was diagnosed in a remote site as metastatic cancer in the lung.
Of the specifically diagnosed malignant STT, leiomyosarcoma formed the commonest followed by rhabdomyosarcoma, Ewing’s/PNET, liposarcoma, Dermato Fibrosarcoma (DFSP), synovial sarcoma and Malignant Periphreal Nerve Sheath Tumour (MPNST). Rare cases included ESS, chondrosarcoma, kaposi sarcoma and alveolar soft part sarcoma. In a study done in the same region on 102 malignant spindle cell tumours, sarcoma group ranked the first, topped by rhabdomyosarcoma followed by leiomyosarcoma and liposarcoma [4]. The European study reported liposarcoma in 21%, fibrosarcoma in 11% and leiomyosarcoma in 10% of study participants [23]. Similar findings have been demonstrated by studies from different countries [24,25,26]. Clinically, 80% of leiomyosarcomas were diagnosed in uterus. Although the morphology was more or less convincible, diagnosis was confirmed by immunoreactivity for desmin, Smooth Muscle Actin (SMA) and H-Caldesmon. Interestingly, in this study a case of recurrent leiomyosarcoma was diagnosed based on the a high proliferative index (Ki-67) because the biopsy was a tiny, tru cut biopsy with few bland looking smooth muscle fibers that were difficult to distinguish from benign leiomyoma as both are positive for Desmin, SMA and H-caldesmon.
Rhabdomyosarcoma formed about 10% of the studied malignant STT. It is well-known that alveolar rhabdomyosarcoma often harbors the typical translocation, however embryonal rhabdomyosarcoma lacks any specific genetic aberration. In the ongoing study, diagnosis of rhabdomyosarcomas was morphological supplemented by immunoreactivity for desmin, myogenin and myoD1. It is worthy to mention that two cases of embryonal rhabdomyosarcoma also expressed CD99. Clinical, radiological and morphological features in addition to negativity for pankeratin stratified them within the rhabdomyosarcomas rather than Rhabdoid tumour where the cells also express desmin, myoD1 and myogenin immunoreactivity [27].
Despite the fact that definite diagnosis of Ewing’s/PNET requires identification of specific chromosomal translocations using fluorescence in situ hybridisation or Polymerase Chain Reaction (PCR) [28], 12 cases were typed as extraskeletal Ewing’s/PNET. Stratifying these cases as Ewing’s/PNET was corroborated with the available clinical data, the morphologic criteria of tumour cells and their immunoreactivity for CD99 and Fli-1 in, addition to PAS positivity/PASD negativity, their lacking CD45 and Terminal Deoxynucleotidyl (TDT) immunoreactivity. Here, it is interesting to say that Ewing’s/PNET was the only soft tissue cancer diagnosed during infancy, where 2/12 cases were below one year of age, and 9/12 (75%) below 20 years of age. In the same line, a large American study enrolling 1631 patient with confirmed Ewing’s/PNET family of tumours, 76.3% of patients were younger than 24 years [29]. As well, it is of great interest that Epithelial Membrane Antigen Transferase (EMA) was positive in three cases of Ewing’s/PNET. Actually speaking, expression of epithelial markers like EMA, doesn’t rule out the diagnosis of Ewing’s/PNET, and the integration of clinical, radiological, morphological, immunohistochemical and molecular findings shall be given in consideration for diagnosis of bone and soft tissue sarcomas, especially in tumours with an unusual immunoprofile [30].
Concerning liposarcoma, the diagnosis is morphological rather than immunohistochemical or molecular. However, two cases were pleomorphic and de-differentiated that needed CDK4 and S-100 protein immunohistochemistry [Table/Fig-13]. Diagnosis was strengthened by immunopositivity for nuclear and cytoplasmic positivity for S-100 protein and nuclear positivity for CDK4 combined with negativity for Melan A, Human Melanoma Black 45 (HMB45) and Desmin and other muscle markers. CDK4 is a recent immune marker, which with positivity for MDM2 (Mouse Double Minute 2) form a synergistic combination required for variably differentiated liposarcoma [31].
A precise diagnosis of DFSP needs molecular identification of the specific t(17;22) translocation. In this study, 11 cases were stratified as DFSP for their characteristic morphology as storiform growth of malignant spindle cells with a high mitotic activity and infiltrative foci within the adjacent subcutaneous fat globules. Morphology was supplemented by diffuse expression of CD34.
The term “synovial sarcoma” was given for six malignant spindle cell cases, based on their strong CD99 immunostaining. It is worth noting that CD99 expression gives a wide carcinomatous, lymphomatous and sarcomatous differential diagnosis [32]. However, stratifying our cases as synovial sarcoma was corroborated with the available clinical data and the morphologic criteria of fibrosarcomatous growth pattern of actively dividing spindle to epithelioid cells. Gland-like spaces were described here and there within the spindle cells resulting in a focally haemangiopericytomatous appearance. This is in addition to other integrated immunomarkers, like vimentin, EMA and Bcl2. In 2008, a case of monophasic synovial sarcoma presented clinically as a foot gouty tophus has been reported in the same region and the specific chromosomal t(X;18) translocation has been confirmed [8].
Positivity for CD99 was also noticed in three other fibrosarcomatous, focally haemangiopericytomatous growth of actively proliferating malignant spindle cell tumour with extensive, focally geographic necrosis. In a single case, a nerve bundle could be detected at the edge of the tumour mass. These cases were typed as MPNST. The diagnosis was corroborated with the immunohistochemical positivity of tumour cells for CD99 and Leu7 (CD57), as well, focally, S-100 protein was positive.
It is interesting to note that out of the three chondrosarcoma cases, the round neoplastic cells of one case also expressed CD99 immunoreactivity. However, the morphologic features of epithelioid cells within variable degrees of cartilaginous compartment, in addition to their immunoprofile (S-100 protein and Leu7 positivity) supported the diagnostic decision.
There were two vascular tumours formed of slit-like vascular spaces within a spindle cell proliferation, with extravasated red blood cells and intracytoplasmic, PAS+/PASD+, hyaline globules. These tumours were positive for CD31, CD34 and D2-40. These cases were diagnosed as kaposi sarcoma. Both cases were demonstrated in elderly males.
Two other malignant uterine spindle cell tumours were diagnosed as endometrial stroma sarcoma for their classical more or less monotonous spindle cell proliferation that closely resembled endometrial stroma. Mitotic figures were variable, and there were prominent thick-walled arterioles. These cases strongly expressed CD10 and oestrogen receptor immunomarkers.
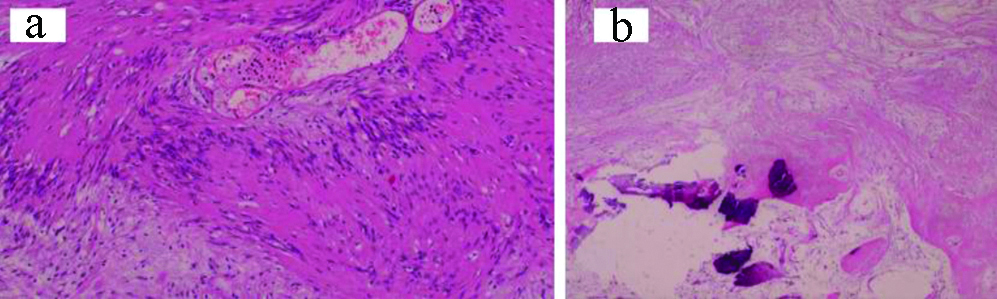
Two fibro/myofibroblastic sarcomas were reported, one as a low-grade fibromyxoid sarcoma while the other as myxofibrosarcoma. As demonstrated in [Table/Fig-16], diagnosis of the low-grade fibromyxoid sarcoma was based on the clinically large, well-defined, irregular thigh mass; microscopically, infiltrative bland looking spindle cells within myxoid background, and focally collagenised stroma. The myxofibrosarcoma was composed of pleomorphic spindle cells with bizarre multinucleated giant cells in myxoid, necrotic background. Some thick-walled blood vessels with surrounding dense rim of inflammatory cells were noted. In addition to Vimentin, both cases also expressed CD99. Mucin 4 (MUC4) was positive in the low-grade fibromyxoid sarcoma. Diagnosis was strengthened by their negative immunoexpression to the specific smooth muscle, neural, melanocytic and adipocytic markers.
The term alveolar soft part sarcoma was given for a neoplasm showing an alveolar growth of non-cohesive large polygonal cells with PAS+/PASD+ eosinophilic granular cytoplasm, vesicular nuclei and prominent nucleoli. No specific immunoprofile could be identified.
Limitation(s)
This is a single town (province) study and these results may not be directly applicable to other areas in Iraq. Following the discovery of recent buildup of highly specific immunomarkers and molecular acumen, diagnostic armamentarium for STT has expanded dramatically. Further, certain defects in these new markers used may have compromised some results.
Conclusion(s)
Benign STT are more common than sarcomas. Diagnosis and classification of STT should be based first on grounds of histologic acumen which is sufficient for most benign tumours and, to some extent, for sarcomas. Undifferentiated sarcomas cannot be categorised without immunohistochemistry. Loss of some differentiation antigens in undifferentiated cancers often necessitate the use of panels of antibodies, and considerable cases even need further evaluation, like molecular studies.
DFSP: Dermatofibrosarcoma protuberans; MPNST: Malignant peripheral nerve sheath tumour; PNET: Primitive neuroectodermal tumour; *PAS/PASD: Periodic acid schiff/Periodic acid schiff/diastase special enzymes used in histochemistry, Bcl-2: B-cell lymphoma 2; CD: Cluster of differentiation; CDK4: Cyclin-dependent kinase 4; EMA: Epithelial membrane antigen; ER: Estrogen receptor; FLI1: Friend leukaemia integration 1; MUC: Mucin; SMA: Smooth muscle actin