Anti-TPO Ab titres are of clinical interest among various autoantibodies elevated in autoimmune thyroid diseases [5]. Population based studies have reported anti-TPO Ab in 16.7% of adult subjects [5]. In asymptomatic subjects, elevated levels of anti-TPO Ab may suggest predisposition to thyroid autoimmune diseases [7]. Perimenopause refers to the period around menopause (40-55 years) [3]. This includes 8-10 years before menopause (when the endocrinological, biological and clinical features of approaching menopause commence) and the first year after menopause. Post-menopause refers to the years after menopause [8]. Symptoms of thyroid disorders often develop so insidiously, that they go unnoticed and there is also the risk of being misinterpreted for menopausal symptoms [9]. Women in this age group are vulnerable to osteoporosis and cardiovascular diseases, added thyroid disorders exaggerate the existing risk; hence it is imperative for timely intervention of this chronic non-communicable disorder amongst this neglected sector of tribal perimenopausal population [7,8].
Literature search has shown that there is a definite paucity of data regarding thyroid function status in perimenopausal women of tribal community. Hence, the aim of this study was to assess the thyroid function and anti-TPO Ab status amongst perimenopausal women by estimating TSH, Total T3, Total T4 and anti-TPO Ab levels in the serum.
Materials and Methods
A descriptive cross-sectional study was carried out in Hakki Pikki tribal colony, eight kilometers from Bengaluru, which comes under the field practice area of Community Medicine, Rajarajeswari Medical College and Hospital (RRMCH), Bengaluru, Karnataka, India. This study was conducted for a period of six months from July 2017 to December 2017. The sample included 41 perimenopausal women in the age group of 40-55 years. The total population of the colony was 420 whereas the total no. of houses in the colony was 105. All perimenopausal women in the age group between 40-55 years were included by doing a house to house survey with the help of Community Health Worker and Government Primary School teacher. Prior permission was obtained from the Ramanagara District Health Officer.
Perimenopausal women with a previous history of thyroid disorder with or without treatment, previous history of thyroid surgeries, without supplementation of iodised salt, were excluded from the study. Ethical clearance was obtained from Institutional Ethical Clearance Committee (dated 08/07/2016). After explaining the study objectives, informed consent was obtained from the study subjects. Five mL of venous blood sample under full aseptic precautions was collected from each participant in collection tubes containing clot activators. Collected samples were brought to the Clinical Biochemistry Laboratory of RRMCH in an insulated carrier. All the samples were processed and analysed on the same day of sample collection. TSH, total T3, total T4 and anti-TPO Ab were estimated by Competitive CLIA [10] in SNIBE Maglumi 1000 fully automated immunoassay analyser using reagent kits supplied by SNIBE Diagnostic, after running Biorad control material.
Procedure as described by Lopez J et al., (the literature provided by the manufacturer in the reagent kit) was followed. The procedure for the estimation of TSH, Total T3, Total T4, FT3, FT4, and anti-TPO Ab was same except that the monoclonal antibody and purified antigen coated on magnetic microbeads depends on the analyte estimated [10]. In brief, N-(4-aminobutyl)-N-ethylisoluminol (ABEI) was used to label monoclonal antibodies, purified antigens to coat magnetic microbeads. The sample, ABEI label and buffer were mixed thoroughly, incubated at 37°C, then further incubated after adding the solution of the magnetic microbeads coated with antigens. The sample and the magnetic microbeads coated with antigens compete for binding the ABEI label, forming an immune-complex. After precipitation in a magnetic field, the supernatant was decanted and followed by a wash cycle. Subsequently, a starter was added to initiate a chemiluminescent reaction. The light signal produced by a photomultiplier within three seconds as Relative Light Units (RLU) was proportional to the concentration of the analyte present in samples.
Reference ranges are subjected to the laboratory where the test is performed. In this study, reference values used were according to the manufacturer’s recommendations. Reference interval of TSH was 0.3-4.5 μIU/mL, FT3 1.21-4.18 pg/mL, FT4 7.2-17.2 pg/mL, Total T3 69-219 ng/dL, Total T4 5.2-12.5 μg/dL and anti-TPO Ab <30 IU/mL [10]. Efficient diagnostic strategies can be based on the initial measurement of serum TSH and differential diagnosis of thyroid diseases requires additional measurement of free T4, free T3 and auto-antibodies [11]. Due to financial constraints, only Total T3 and Total T4 was estimated in all perimenopausal women and FT3 and FT4 were estimated only when Total T3 and Total T4 values were abnormal. Based on Thyroid Function Test (TFT) profile, the thyroid status was broadly classified into hypothyroidism, hyperthyroidism and euthyroidism. Hypothyroidism was further classified into SCH and overt hypothyroidism [6,10]. Hypothyroidism was classified as clinical if TSH was >4.5 μIU/mL, Total T3 <69 ng/dL and Total T4 <5.2 μg/dL. SCH if TSH was > than 4.5 μIU/mL with normal serum Total T3 and Total T4 level [6,10]. Hyperthyroidism was classified as clinical if TSH was <0.1 μIU/mL, Total T3 >219 ng/dL, Total T4 >12.5 μg/dL, subclinical hyperthyroidism if TSH was <0.1 μIU/mL with normal serum Total T3 and Total T4 levels [6,10]. Study participants were considered euthyroid if the Total T3, Total T4 and TSH levels were within the reference range. Based on the anti-TPO Ab level, study subjects were classified into anti-TPO Ab positive (anti-TPO Ab >30 IU/mL) and negative (anti-TPO Ab <30 IU/mL) [12].
Statistical Analysis
Open Epi version 3 was used to find out Mean±SD of TSH, T3, T4 and anti-TPO Ab. ‘Student’s t-test’ was employed to test the difference between the mean values with significant set at p<0.05. Pearson’s correlation coefficient (r) was estimated to measure the strength of a linear association between anti-TPO Ab and TFT parameters, where the value r=1 means a perfect positive correlation and the value r=-1 means perfect negative correlation. Nearer the value is to zero, the weaker is the relationship.
Results
A total of 41 perimenopausal women were investigated. The mean age of these women was 46.37±3.6 years. Majority of the them were in the age group of 40-45 years [Table/Fig-1].
Age distribution of perimenopausal women.
| Age in years | Perimenopausal women n=41 | % |
|---|
| 40-45 | 21 | 51% |
| 46-50 | 17 | 42% |
| 51-55 | 3 | 7% |
Mean age: 46.37 years SD: 3.6 years
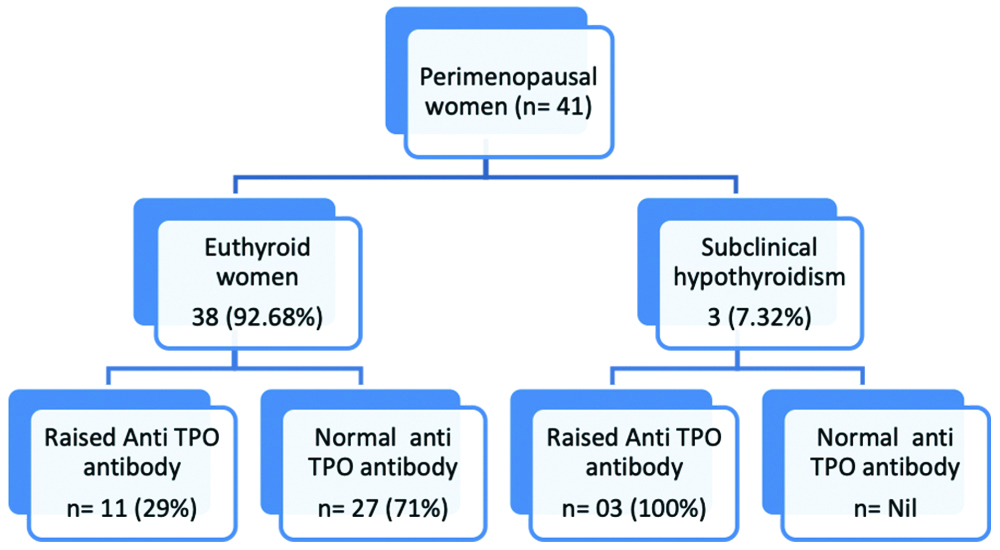
Biochemical evidence of SCH in 3 (7.32%) women and euthyroid status in rest of the 38 (92.68%) women was observed [Table/Fig-2].
Thyroid function status in perimenopausal women and anti-TPO Ab status in different thyroid groups (Based on reference interval of anti TPO Ab: Normal TPO <30 IU/mL, raised >30 IU/mL) [12].
The mean TSH, Total T3, Total T4 and anti-TPO Ab values of the study subjecs are shown in [Table/Fig-3]. Mean TSH, Total T3, Total T4 values of the study subject were within the reference interval whereas anti-TPO Ab titres were more than the reference value.
Serum levels of thyroid hormones, TSH and anti-TPO Ab in perimenopausal women.
| Thyroid profile | Serum levels (Mean±SD) |
|---|
| TSH (μIU/mL) | 2.20±1.87 |
| Total T3 (ng/dL) | 124.63±22.32 |
| Total T4 (μg/dL) | 7.51±1.28 |
| Anti-TPO antibodies (IU/mL) | 128.73±212.67 |
Total T3 and T4 were in normal range
High anti-TPO Ab titre was observed in all the three women with SCH though the TSH level was mildly elevated [Table/Fig-4].
Serum levels of study variables in three subclinical hypothyroid women.
| SCH women | TSH (μIU/mL) | Total T3 (ng/dL) | Total T4 (μg/dL) | Anti-TPO Ab (IU/mL) |
|---|
| No.1 | 6 | 114.8 | 6.6 | 463.2 |
| No.2 | 7.5 | 137 | 6.6 | 517.4 |
| No.3 | 10.3 | 121.6 | 5.8 | 768.2 |
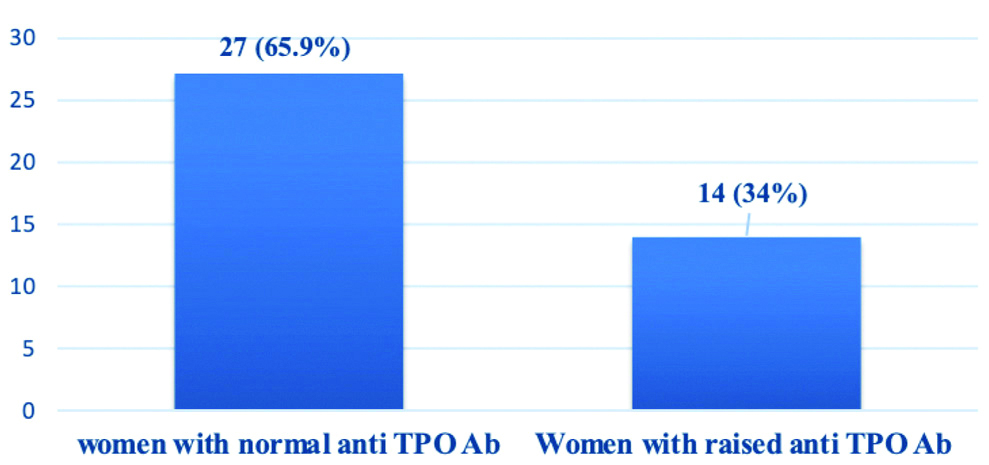
Normal anti-TPO Ab titres was noted in 27 women, raised anti-TPO Ab titre was recorded in 14 women [Table/Fig-5], among them 3 (21%) had SCH and remaining 11 (79%) were euthyroid [Table/Fig-2].
Anti-TPO Ab status of tribal perimenopausal women.
Among these 14 anti-TPO Ab positive women, only one woman had TSH level >10 μIU/mL, two women had >5 μIU/mL and rest of the 11 women had TSH within the reference range.
Significant difference (t statistic-8.80, df (degrees of freedom)-3.9, p=<0.001) in anti-TPO Ab level was observed in women with normal and raised anti-TPO Ab [Table/Fig-6].
Comparison of anti-TPO Ab level in women with normal and raised anti-TPO Ab.
| Normal anti-TPO Ab n=27 | Raised anti-TPO Ab n=14 |
|---|
| anti-TPO Ab Mean±SD (IU/mL) | 6.92±5.21 | 363.66±212.98 |
| t statistic-8.80, df (degrees of freedom)- 3.9 **p<0.001 |
**significant set at p<0.05
Significant difference {t statistic-2.35, df (degrees of freedom)-12, p=0.03} in anti-TPO Ab level among euthyroid and SCH women was noted [Table/Fig-7].
Comparison of anti-TPO Ab level among euthyroid and subclinical hypothyroid women.
| Euthyroid women n=11 | Subclinical hypothyroid women n=03 |
|---|
| anti-TPO Ab Mean±SD (IU/mL) | 303.85±190.34 | 582.93±132.85 |
| t statistic-2.35, df (degrees of freedom)-12, p=0.03* |
*significant set at p<0.05
The difference between the means of anti-TPO Ab levels among the groups was statistically significant as the p is <0.05 and no significant difference in TSH, Total T3 and Total T4 levels among normal and raised anti-TPO Ab subjects [Table/Fig-8] was noted.
Comparison of TFT levels in women with normal and raised anti-TPO Ab.
| TFT | Normal anti-TPO Ab n=27 | Raised anti-TPO Ab n=14 | *p-value |
|---|
| TSH (0.3-4.5 μIU/mL) | 2.14±1.58 | 3.26±2.85 | 0.11 |
| Total T3 (69-215 ng/dL) | 124.72±25.16 | 120.24±18.71 | 0.561 |
| Total T4 (5.2-12.5 μg/dL) | 7.56±1.3 | 7.04±0.94 | 0.186 |
*p-value is set <0.05 as significant
Significant positive correlation (r=0.7023, p=0.005) was found between anti-TPO Ab and TSH in high anti-TPO Ab positive subjects [Table/Fig-9].
Correlation between levels of anti-TPO Ab and TFT in positive anti-TPO Ab women.
| TFT | ‘r’ value | p-value |
|---|
| TSH | 0.7023 | 0.005* |
| Total T3 | -0.4076 | 0.15 |
| Total T4 | -0.2778 | 0.33 |
Significant positive correlation (‘r’ value is positive and near to 1) was observed between anti-TPO antibodies and TSH in raised anti-TPO Ab subjects. p-value is set <0.05 as significant
Discussion
Thyroid function and gonadal axes are related throughout a woman’s reproductive period. The levels of oestrogen hormone, that enhances thyroid function decreases as the age advances, hence the thyroid function also goes down. This is one of the main reasons stated for increased thyroid dysfunction in perimenopausal women [13]. The present study is a tribal community-based study of thyroid function amongst perimenopausal women. SCH was observed in (7.32%) women. There were no cases of hyperthyroidism in the study population. The most commonly encountered thyroid disorder is hypothyroidism and SCH is approximately 14 times more common than overt hypothyroidism [14] and more so in women of perimenopausal age group [15].
Skaria LK et al., conducted a study on Tribal Women of Baster Region of Chhattisgarh in the age group of 10-80 years. They reported SCH in 10.2% of perimenopausal women which is comparable to the present study. In their study, they also observed other thyroid disorders including overt hypothyroidism (59%) and hyperthyroidism (1%). The attributable reasons for increased occurrence being; hospital-based study, consumption of goitrogenic foods by Baster residents and erosion of iodine rich soil due to heavy rain fall [16].
Satyanarayana PVV and Anand A conducted a large community based prospective study in the East Godavari tribal women in the age group of 15 to 65 years. A total of 316 (36.57%) women were diagnosed as SCH; among them 90 (10.41%) were between 46 and 65 years of age, which can be compared with present study. They concluded that, hypothyroidism is the commonest thyroid disorder even in this post-iodisation period [17]. Perimenopausal women with history of adequate iodine intake are also vulnerable to hypothyroidism as the ability of thyroid gland to take up iodine diminishes as the age increases [8]. So, advancing age has been implicated as one of the main deciding factors in the occurrence of hypothyroidism in this age group [8].
A study in tribal adult population by Arakeri S and Vasu G showed hypothyroidism as the commonest (19%) thyroid disorder in the age group of 41 to 50 years with female preponderance [18]. Valiyaparambil PP et al., studied thyroid disorders in tribal adult population residing in a hill station of Kerala state and compared with non-tribal population [19]. Interestingly, they reported goitre associated thyroid hyperfunction in tribal population and did not report any case of hypothyroidism [19]. The authors opined that this observation may be due to the fact that, whenever iodine deficiency is corrected, thyroid hyper function is expected [19]. Montey et al., (Unpublished data), Department of Biochemistry, RRMCH, observed 22 (21%) urban perimenopausal women with SCH out of 148 perimenopausal women visited the RRMCH, Bengaluru, Karnataka, India. In a southern India region-based study, Latha P et al., reported 17% of SCH amongst urban perimenopausal woman (40 to 55 years, n=60) [14]. The high SCH may be due the fact that these were hospital-based studies with comparatively high sample sizes.
Unnikrishnan AG et al., carried out epidemiological study in general adult population of eight cities of India, they recorded overall 8.02% cases (5.35%-11.8%) of hypothyroidism, with 7.27% in Bengaluru and they also observed increased SCH with increasing age [9]. Even in a large population-based study by Hollowell JG et al., commonest (26%) thyroid disorder reported in both perimenopausal and menopausal women was SCH [20]. The differences in the thyroid function status prompt us to find out the various aetiological risk factors namely geographic differences, ethnicity, occupation, dietary habits, genetic predisposition [21]. Therefore, early detection of SCH in perimenopausal women has to be done as the rate of progression to overt hypothyroidism is 5-18% [22-24].
The TSH test is very sensitive, clearly describes various thyroid disorders and helps the clinician to adjust precise dosage of thyroid replacement therapy [6]. Anti-TPO Ab are considered to be a sensitive marker of autoimmune thyroid disease [25]. Elevated TSH is considered to be the commonest cause of autoimmune thyroid disease in the post-iodisation era [25]. After the sixth decade of life, antithyroid antibodies can be detected in 80% of patients with SCH [25]. Higher levels of antibodies can be detected in persons with a serum TSH level between 3.0 and 5.0 μIU/L with a higher rate of progression to clinical thyroid disease [26]. Elevated anti-TPO Ab are not only observed in Hashimoto’s thyroiditis and Grave’s disease, also seen in 10% of the asymptomatic individuals, this finding in asymptomatic individuals may give a clue for the development of autoimmune thyroid diseases in future [6].
In the present study, 14 (34%) women showed raised anti-TPO Ab, among them 11 (79%) were in euthyroid state and 3 (21%) had SCH. TSH levels of these SCH patients were not more than 10 IU/mL but the anti-TPO Ab levels were very high. This finding is crucial, since the previous studies suggest that raised anti-TPO Ab identifies an autoimmune aetiology for thyroid disorders in post-iodisation era and predict a higher risk of developing overt hypothyroidism [6,7]. Siriwardhane T et al., in their retrospective analysis of clinical data and test results of thyroid markers, showed that the thyroid autoantibodies precede subclinical/overt hypothyroidism and hyperthyroidism and opined that it may be beneficial to consider testing for anti-TPO in conjunction with the primary thyroid markers, TSH and FT4, to prevent long-term morbidity [21]. In present study, raised anti-TPO autoantibodies were also observed in euthyroid women. This finding suggests that, anti-TPO shall be measured along with thyroid hormones while screening for thyroid disorders.
Arakeri S and Vasu G conducted a cross-sectional study to correlate anti-TPO Ab levels with thyroid function status in general population of tribal community in a hilly area of Wayanad, Kerala, India. They witnessed raised anti-TPO Ab levels in 57% of tribal population, not only in SCH {27 (47%)} but also in euthyroid {21 (37%)} and hyperthyroid {9 (16%)} subjects. In their study they also observed high anti-TPO Ab positivity in female tribal women (81%) compared to males (19%) [18]. Unnikrishnan AG and Menon UV reported high anti-TPO Ab positivity in females (26%) than in males (16.81%) and highest anti-TPO Ab positivity (26.86%) in females in the age group of 46-54 years. They also observed anti-TPO Ab positivity in 17.42% of Bengaluru urban general population [4]. Mahanta A et al., in their study in general urban population observed increased anti-TPO Ab in 53% of SCH [27]. Deshmukh V et al., reported anti-TPO Ab positivity in 23.6% of euthyroid and 47.6% of SCH subjects of general urban population [28]. Endocrine Module (PYPP 5260) mention that elevated levels of anti-TPO Ab are found in virtually all cases of Hashimoto’s thyroiditis and 85% of Graves’ disease and 10% of asymptomatic individuals [6]. Cause for high anti-TPO Ab positivity in these perimenopausal women is unclear; this may be due to complex interplay of exogenous factors like infections, iodination, environment, occupation and endogenous factors like immunological factors, genetic, hormonal and ethnic variations [29].
Euthyroid individuals with elevated levels of anti-TPO may suggest a predisposition to thyroid autoimmune disease [6]. Hence, 14 subjects (34.14%) with elevated anti-TPO Ab in present study need to be followed-up as the risk of thyroid disorder is more in these subjects. American Thyroid Association recommends thyroid screening of both genders in the age group of 35 years and then after every five years for early treatment of SCH [22].
Limitation(s)
Free T3 and Free T4 were not estimated in perimenopausal women. Although the entire Hakki Pikki colony was screened for the perimenopausal women, the sample size was small.
Conclusion(s)
This is the first report regarding thyroid function and anti-TPO Ab status in tribal perimenopausal women in Hakki Pikki colony. Anti-TPO Ab positivity was seen in 34% women; Out of them majority (79%) were in euthyroid state and remaining (21%) had SCH. This observation of raised anti-TPO Ab levels in this vulnerable age group suggest autoimmune aetiology and these women deserve strict vigil as they are likely to progress to hypothyroid state. Hence, in addition to routine thyroid profile, Authors suggest estimation of anti-TPO Ab in these women. Early diagnosis of thyroid disorders among this subset of women also helps to differentiate perimenopausal symptoms from thyroid dysfunction thus preventing the morbidity associated with this disorder.
Regular screening of perimenopausal women for thyroid disorders by performing TFT along with anti-TPO Ab. Need of the hour is to follow-up subjects with SCH and euthyroid women with elevated anti-TPO Ab as they are more prone to develop overt thyroid disease.
Mean age: 46.37 years SD: 3.6 years
Total T3 and T4 were in normal range
**significant set at p<0.05
*significant set at p<0.05
*p-value is set <0.05 as significant
Significant positive correlation (‘r’ value is positive and near to 1) was observed between anti-TPO antibodies and TSH in raised anti-TPO Ab subjects. p-value is set <0.05 as significant