Achromobacter is a non-fermenting Gram Negative Bacillus (GNB) belonging to the family Alcaligenaceae. It is emerging as a pathogen, causing myriad of infections in different patient groups. Often wrongly identified by conventional microbiological techniques, Achromobacter when recovered from clinical specimens have often been discarded as contaminants. Accurate species identification of the currently known 21 species of Achromobacter, is difficult, labour-intensive and time consuming. Automation has made it possible to reliably and correctly identify Achromobacter species and other non-fermenters. Matrix Assisted Laser Desorption Ionisation-Time Of Flight-Mass Spectrometry (MALDI-TOF-MS) is a boon as it rapidly and accurately identifies Achromobacter species in clinical specimens. Three cases of Achromobacter infection, highlighting the pathogenic potential of the bacteria and the role of automation in its identification are presented with due consent from relatives or patient themselves. This case series highlights that Achromobacter when recovered from clinical specimens should not be discarded as contaminants especially if isolated repeatedly. Case one was a patient who had developed pyelonephritis and sepsis, associated with bacteraemia and ended fatally, Achromobacter was recovered on two occasions from the blood culture. Case two was a patient who was diagnosed of acute pancreatitis with an infected peripancreatic collection who recovered with prolonged therapy, Achromobacter was repeatedly isolated from the pus draining from the peripancreatic collection. Case three was a patient with an infected malignant ulcer with purulent discharge from the swelling. In all three cases Achromobacter was isolated and identified by MALDI-TOF-MS.
Achromobacter xylosoxidans/denitrificans, Acute pancreatitis, Bacteraemia, Pyelonephritis, Urosepsis
Introduction
Achromobacter species including both Achromobacter xylosoxidans subsp. xylosoxidans (A. xylosoxidans) and Achromobacter xylosoxidans subsp. denitrificans (A. denitrificans) are non-fermenting, aerobic, motile, GNB. In nature, they are found in aquatic environments and soil. In the human body, A. xylosoxidans inhabits the respiratory tract, gastro-intestinal tract and the ear canal. Phenotypic similarity and taxonomic complexity preclude their accurate identification by conventional phenotypic microbiological methods [1]. Due to this, they often get discarded as contaminants; however their pathogenic potential has been clearly recognised now. Achromobacter is identified accurately by the VITEK MALDI-TOF MS, or VITEK MS automated system (bioMerieux, France) [2]. Presently 21 species of Achromobacter have been identified by genome sequencing. Both phenotypic and genotypic methods for identification of clinically important Achromobacter species are available [3]. Currently, the VITEK MS reference database is able to identify Achromobacterxylosoxidans and denitrificans, rapidly and accurately by analysing the protein mass spectrum which is a boon for clinical microbiology laboratories [4]. This group of bacteria has emerged as opportunistic pathogens in immunocompromised hosts, and those having a structural anatomical defect, abnormality or an indwelling catheter or device [5]. They are similar to Pseudomonas species and have been implicated in nosocomial outbreaks associated with contaminated fluids [6]. Achromobacter has been isolated from blood in septicaemia and septic shock [7,8], from the peritoneal fluid in peritonitis [9], from pleural fluid in cases of empyema, from urine in cases of Urinary Tract Infections (UTI) [10], wound exudates [11] and respiratory secretions in cystic fibrosis [12]. It has rarely been associated with acute pancreatitis [13-15], lymph node [16] and infection of cancerous ulcer [17]. Three cases of Achromobacter infection, identified with MALDI-TOF VITEK MS are reported here.
Case Series
Case 1
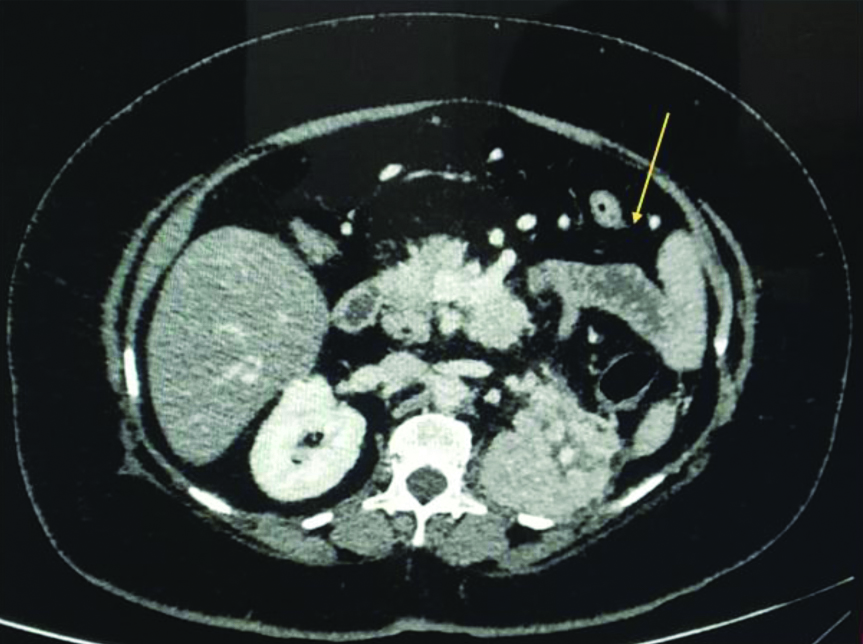
A 38-year-old male with no known co-morbidities presented to a Peripheral Hospital with 10 days history of high-grade fever with chills and rigors, bilateral loin pain radiating to the back and urinary complaints. Patient visited a local dispensary for his complaints of fever and pain abdomen with no relief, details of treatment not known. He then came to the peripheral hospital where on initial evaluation he was admitted as a case of UTI. His vital parameters were recorded as temperature-101°F, pulse-110/minute, blood pressure-106/60 mmHg and respiratory rate of 28/minute. On abdominal examination, he had bilateral flank tenderness. His blood picture revealed microcytic hypochromic anaemia, Haemoglobin (Hb)-10.9 gm/dL, Total Leucocyte Count (TLC) of 3.0×103 cells/μL, fever panel including malaria and dengue serology were negative, chest radiograph was normal. Ultrasonography and urine culture were not done at the time of admission; however, his routine urinalysis was normal. His Blood Urea Nitrogen (BUN) was 25 mg/dL, creatinine was 0.9 mg/dL, total bilirubin was 0.8 mg/dL, aspartate aminotransferase was 32 IU/L and alanine aminotransferase was 41 IU/L, all within normal limits. With a provisional diagnosis of UTI, he was started on antibiotics; ceftriaxone 1-2 g IV/day and levofloxacin IV 750 mg/day. On the second day in hospital despite therapy he continued to be febrile and started complaining of breathlessness. He was given oxygen supplementation by a nasal cannula to maintain his SpO2 of 94% at 4 L/minute. By the third day in view of his worsening condition he was transferred to a larger facility with a suspicion of urosepsis. On initial examination at the Tertiary Care Hospital, the patient looked toxic. Preliminary investigations revealed microcytic anaemia with Hb-10.6 gm/dL, polymorphonuclear leucocytosis with TLC-15×103 cells/μL with 85% neutrophils. Patient was immediately started on meropenem IV 1 g every 12 hours while his levofloxacin IV 750 mg/day was continued. His blood and urine samples were sent to the microbiology laboratory for bacteriological culture and antibiotic sensitivity. Radiological imaging of the abdomen revealed bulky kidneys with left-sided pyelonephritis [Table/Fig-1].
Contrast-enhanced computed tomography of abdomen of case 1 showing bulky right kidney and extensive loss of corticomedullary differentiation in left kidney (arrow), Impression: Acute Pyelonephritis (L).
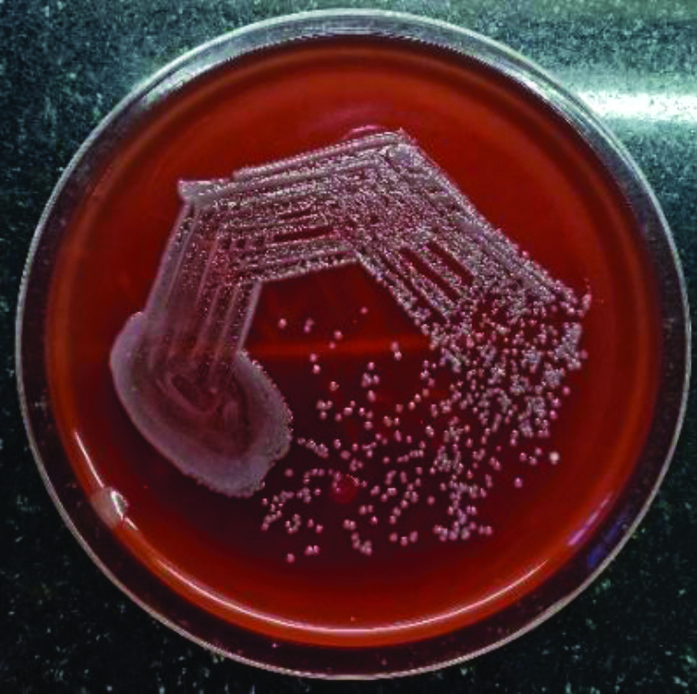
We received a single blood culture bottle for culture at the microbiology laboratory which was flagged positive within 24 hours by the BacT ALERT automated blood culture system. On sub-culturing the broth, 1-2 mm in size, pale pink Non-Lactose Fermenting (NLF) colonies grew on the MacConkey (MA) agar on aerobic incubation at 37°C for 24 hours. The Blood Agar (BA) revealed small, greyish non-haemolytic colonies [Table/Fig-2a,b]. Gram staining revealed a GNB which was motile, positive for catalase and cytochrome oxidase test. Conventional biochemical tests revealed that the organism did not ferment sugars. It was identified as A. xylosoxidans by MALDI-TOF MS, VITEK MS automated system (bioMerieux, France). Since a single blood culture sample had been received, the isolate was discarded as a contaminant. However, paired blood cultures were asked for in accordance with the blood culture protocols. His urine culture was sterile and did not show any growth.
(a) Non-haemolytic, translucent colonies of Achromobacter on blood agar.
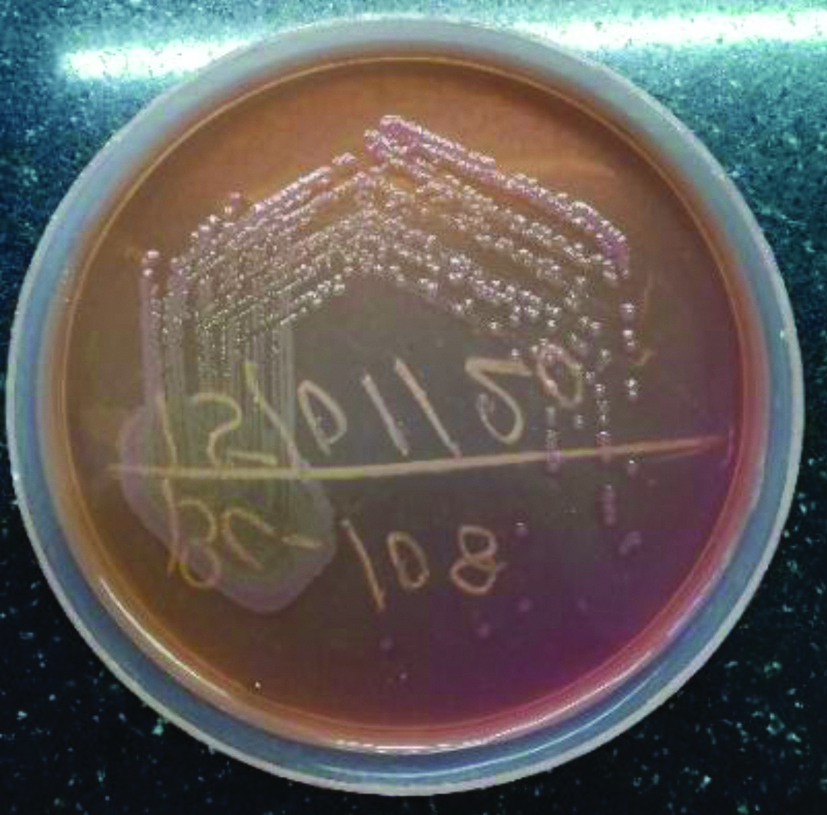
Non-lactose fermenting colonies of Achromobacter on MacConkey agar.
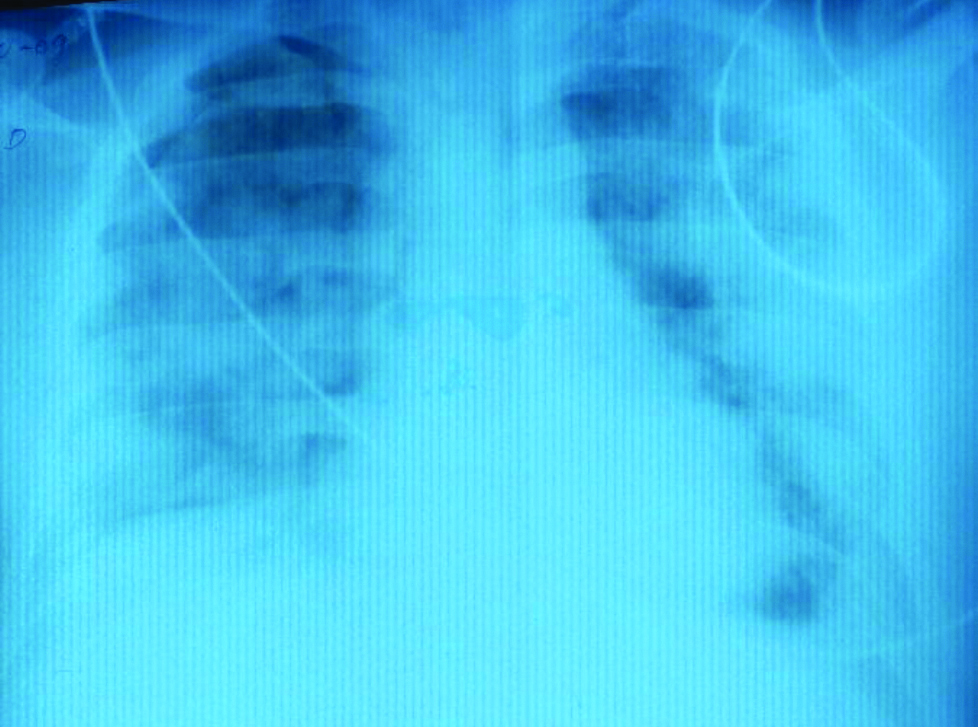
After an initial improvement, the patient’s condition started to deteriorate, he became hypoxic and febrile. His raised serum procalcitonin levels of 1.17 ng/mL and leucocytosis revealed that the patient had progressed to sepsis. Arterial blood gas analysis showed PaO2/FiO2 less than 300 indicating acute respiratory distress. Chest radiograph showed bilateral diffuse fluffy alveolar opacities [Table/Fig-3]. Teicoplanin 400 mg every 12 hours with renal modification was added to his antibiotic regimen, but he had a downhill course and became hypotensive requiring inotropic support.
Chest radiograph posteroanterior (PA) view of case 1 showing bilateral fluffy opacities in both lungs suggestive of Acute Respiratory Distress Syndrome (ARDS).
In the laboratory, his repeat paired aerobic blood cultures flagged positive in the BacT/ALERT (bioMerieux) automated blood culture system within a few hours. On sub-culturing the broth on BA and MA agar, non-haemolytic and NLF colonies grew which were identified as A. xylosoxidans by MALDI-TOF MS, VITEK MS automated system (bioMerieux, France). Antibiotic sensitivity testing was performed both manually by disk diffusion method. As well as by automated VITEK 2 system and susceptibility break points were interpreted as per the Clinical Laboratory Standards Institute (CLSI) guidelines for other non-fermenters; non-enterobacteriaceae [18]. Despite best efforts, the patient had a fatal end.
Case 2
A 24-year-old male with no co-morbidities was admitted to a peripheral hospital with complaints of pain in the upper abdomen radiating to the back and vomiting for two days. There was no history of fever, loose stools, trauma, recent drug intake or history of similar episodes in the past. On examination, he was afebrile, anicteric and his abdomen was tense and distended. He was admitted with a provisional diagnosis of acute pancreatitis. On investigation, he had a high TLC 17×103 cells/μL with 95% neutrophils and raised serum amylase 3018 U/L, creatinine 1.0 mg/dL and random blood sugar were normal. His serum calcium was low and his lipid profile was normal. A contrast enhanced computed tomography scan of his abdomen revealed evidence of acute necrotising pancreatitis with ascites [Table/Fig-4]. There was no evidence of cholelithiasis. A final diagnosis of acute necrotising pancreatitis was made and the patient was treated empirically as per the guidelines [19,20]. He was started on meropenem IV 2 gm every six hours according to the hospital antibiotic policy for abdominal infections. After two days he started becoming febrile with high spiking fever in the range of 102-103°F indicating an infective focus. However, his blood culture did not yield any growth. He underwent insertion of pig tail catheters to drain out the peripancreatic collection followed by an exploratory laparotomy. His pancreatic fluid was sent for bacterial culture. Growth obtained after 24 hour of incubation on BA, revealed small, non-haemolytic colonies, and on MA agar pale NLF colonies. They were catalase and oxidase positive, gram negative bacilli. On routine biochemical testing the isolate did not ferment sugars; further identification by MALDI-TOF MS identified the organism as A. xylosoxidans. Antibiotic sensitivity by VITEK-2 system was done using GN and N90 cards. We received drain fluid and two pus samples over the next 10 days, all grew Achromobacter. The isolates had the same sensitivity pattern; sensitive to piperacillin-tazobactam, resistant to meropenem, amikacin, ceftazidime. As identified by the antibiogram, the patient was treated with IV piperacillin-tazobactam 4.5 g every eight hourly and IV levofloxacin 500 mg for 14 days. Haematological and biochemical parameters along with the clinical picture improved drastically subsequent to the appropriate antimicrobial therapy over next few weeks. The patient was discharged after almost four months in the hospital and recovered.
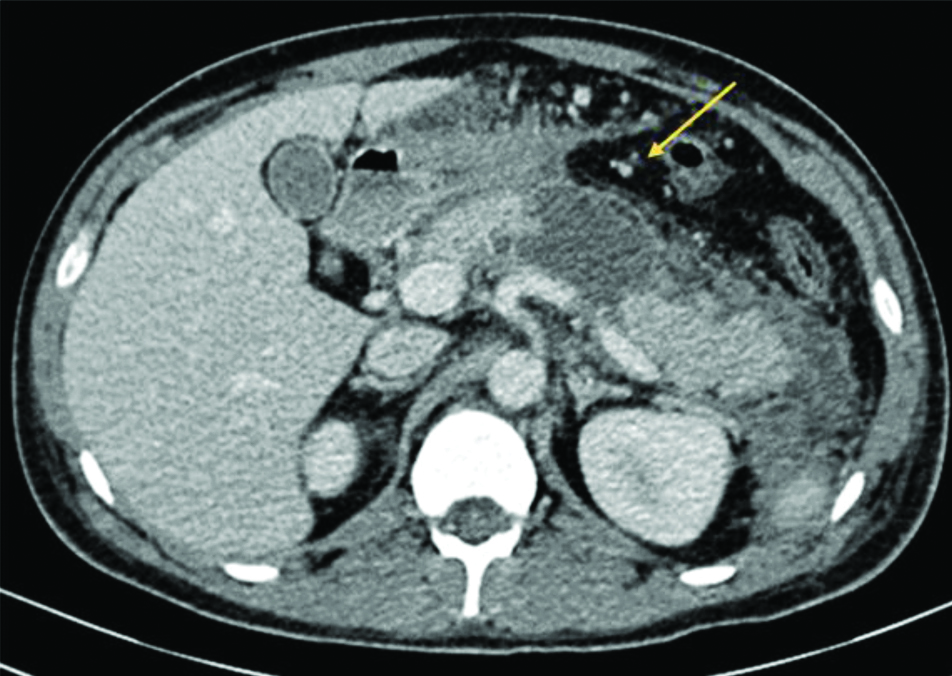
Contrast-enhanced computed tomography of abdomen of case 2 showing oedematous pancreas with acute necrotic collections (arrow) within the body of pancreas and peripancreatic region. Significant perinephric stranding seen. Impression- Acute Necrotising Pancreatitis with Modified CT Severity Index 08/10.
Case 3
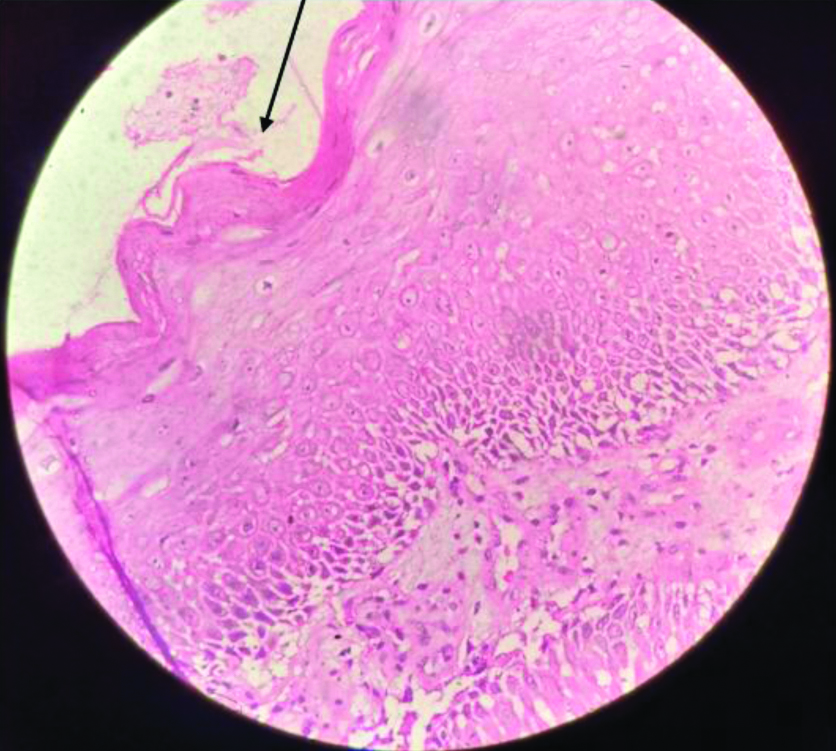
A 42-year-old female presented with a swelling on the right side of the groin of four months duration. The swelling was insidious in onset, 3×2 cm non-progressive, associated with mild pain. Twenty days later the patient noticed a purulent discharge from the swelling. She took some over the counter oral medications (details not known) with partial resolution of the pus discharge; however, the swelling did not reduce. There was no history of fever, weight loss or night sweats. On examination, there was a 3×2 cm solitary swelling situated in the right groin which was firm in consistency and non-tender. Purulent discharge was oozing from the swelling. The surface of the swelling was hyperpigmented, verrucous and rough. A differential diagnosis of cutaneous tuberculosis and/or a fungal infection was made. An incisional biopsy from the swelling in the right groin was taken and sent for histopathological examination and the draining pus was sent for bacterial culture. The pus from the swelling was cultured on BA and MA agar. NLF colonies grew which were identified as Achromobacterdenitrificans by MALDI TOF. The tissue was stained for fungal elements by the Grocotts stain and for mycobacteria by the Ziehl Neelsen (ZN) stain, both were negative. Skin biopsy revealed islands of moderately differentiated squamous cells [Table/Fig-5]. A diagnosis of infected malignant ulcer with an underlying moderately differentiated squamous cell carcinoma was made. The patient was treated as an outpatient with oral ciprofloxacin 500 mg twice a day for 10 days according to the antibiotic sensitivity report, which led to the resolution of the pus discharge. The patient is undergoing treatment for the malignancy and is on follow-up.
Haematoxylin and eosin stained biopsy specimen of Case 3. Arrow showing ulcerated epithelium with underlying squamous epithelium cells which are moderately differentiated. (magnification-40x) Impression- Ulceration with underlying moderately differentiated squamous carcinoma.
In all patients, Achromobacter was isolated on multiple occasions, the summary and treatment details of all three patients has been tabulated [Table/Fig-6].
Summary of all three cases.
| Case | Age (years)/Gender | Diagnosis | Haematological findings | Clinical/Biochemical findings | Antibiogram | Treatment | Outcome |
| 1 | 38/M | Acute Pyelonephritis with Urosepsis | Hb=10.6 g/ dL; WBC count=15.0×103 cells/ mL with 85% neutrophils | Hyperthermia (39.2°C), Tachycardia (110/minute), hypotension (106/60 mmHg) | Sensitive to Cefoperazone-sulbactam, imipenem, meropenem. Resistant to ciprofloxacin, levofloxacin, aminoglycosides | Intravenous meropenem 1 gm every 12 h and Injection levofloxacin, IV 750 mg/day.Later Teicoplanin 400 mg every 12 h with renal modification was added. | Patient died |
| 2 | 24/M | Acute Necrotising Pancreatitis with infected peripancreatic collections | Hb=10.2 g/dL; TLC=17.0×103 cells/mL 95% Neutrophils | Grossly deranged liver and kidney function tests, Serum Amylase-3018 U/L, creatinine-1.0 mg/dL | Sensitive to Cefoperazone-sulbactam, levofloxacin, piperacillin-tazobactam. Resistant to aminoglycosides, ceftazidime, imipenem, meropenem | Intravenous meropenem 2 gm 6 hourly later changed to Piperacillin/tazobactam 4.5 g IV every 8 hourly for 14 days plus, levofloxacin IV 500 mg once daily for 14 days. | Patient was discharged after a prolonged stay in hospital. |
| 3 | 42/F | Infected Malignant ulcer (Rt Groin) | Hb=9.2 g/dL; TLC=13.4×103 cells/mL | Liver and kidney function tests within normal limits | Sensitive to cefepime, ofloxacin, piperacillin/tazobactam, levofloxacin, ciprofloxacin, imipenem, ceftazidime cefoperazone-sulbactam, imipenem, trimethoprim-sulfamethoxazole | Treated with oral Ciprofloxacin 500 mg twice a day and local wound dressings with resolution of pus discharge | Patient is on follow-up for malignancy |
Discussion
A. xylosoxidans was first described from the human ear canal in 1971 by Yabuuchi E and Ohyama A [21]. Phenotypic similarity and taxonomic complexity preclude their accurate identification by conventional microbiological methods. Achromobacter causes a wide spectrum of diseases. Here, three cases were highlighted. Case 1 was bacteraemia and urosepsis due to A. xylosoxidans subsequent to pyelonephritis. The organism has been associated with urological abnormality or stones which was also seen in the patient during investigations. Though patient one initially reported with urinary symptoms but due to lack of bacteriological culture facilities, urine culture could not be done thus, losing an important diagnostic clue. Thereafter, urine culture done at the tertiary centre was sterile possibly due to the antibiotic therapy. The infection was fulminant and bacteraemia due to A. xylosoxidans was detected on two separate occasions. Isolation of Achromobacter on multiple occasions can be due to the adaptation of the bacteria to the host environment, promoting selection of hypermutable strains. An isolate of A. xylosoxidans can easily be mistaken for a non P.aeruginosa strain of Pseudomonas or for a strain of the Burkholderia cepacia complex, but the unusual susceptibility pattern suggests the correct identity. Methods for susceptibility testing are not standardised. Piperacillin tazobactam and carbapenems are active in vitro and are suggested as appropriate initial therapy for serious A. xylosoxidans infections, whereas trimethoprim-sulfamethoxazole may be used for urinary tract and other infections not requiring parenteral therapy. Although the Achromobacter strains are generally resistant to other cephalosporins, aminoglycosides, aztreonam and narrow spectrum penicillins but ceftazidime shows activity against these strains. Susceptibility to fluoroquinolones is variable and high rates of resistance to ciprofloxacin and aminoglycosides have been noted in strains isolated from cystic fibrosis patients. High concentrations of colistin inhibit most strains. Resistance mechanisms include a constitutive oxacillinase and acquired β-lactamases, as well as multidrug efflux pumps. Various case reports recommend A. xylosoxidans is susceptible to co-trimoxazole, ureidopenicillins, carbapenems and β-lactam combination agents [22]. Case 2 highlights pyogenic infection due to Achromobacter which was isolated from an infected peripancreatic collection in acute pancreatitis. The infection only resolved after prolonged antibiotic therapy and a surgical necrotomy. Case 3 highlights the ability of this bacteria to infect malignant swelling. The case series highlights the important role of MALDI-TOF-MS in identification of Achromobacter species rapidly and accurately. The non-fermenters commonly isolated and reported by a microbiology laboratory are limited to Pseudomonas aeruginosa and Acinetobacter baumanii. This might be due to unfamiliarity with identifying non-fermenters routinely by conventional techniques [23]. Species level identification of other non-fermenters is difficult [24]. Availability of automated systems like VITEK MS and VITEK has helped in early identification and timely treatment [25]. However, VITEK requires the bacterial inoculum to be introduced into a card in the VITEK 2 Compact automated identification system and further, incubated for approximately six hours. VITEK MS MALDI-TOF is increasingly being used in routine microbiology diagnostics for rapid and accurate identification of bacteria up to various levels; genus, species or subspecies. Briefly the process entails mixing the bacterial colony to be identified by coating it with an energy absorbent matrix. The matrix along with it the sample which is within the matrix is dried and crystallised. The sample is then subjected to a laser beam. Desorption and ionisation with the laser beam produces protonated ions from analytes such as proteins in the sample. These proteins remain intact with predominantly a single positive charge in the gas phase. In an electric field, the times-of-flight of charged proteins along a tube held at high vacuum after acceleration are proportional to the square root of the mass-over-charge ratios for the proteins hence, allowing a mass spectrum to be generated, which can then be used to identify the proteins in the sample. These charged analytes are detected and measured using a TOF analyser. A characteristic Peptide-Mass-Fingerprint (PMF) is generated for analytes in the sample based on the TOF information. Microbial identification by MALDI-TOF is performed by comparing the PMF of unknown organism with PMFs available in the database [26].
Conclusion(s)
The cases reported by the authors highlight the necessity of routine identification of Achromobacter species which are often confused with other non-fermenters and discarded as environmental contaminants. Correct identification of the organism affects the clinical management as targeted therapy improves patient outcome. Automation has paved the way for identifying organisms which may be difficult to identify by the conventional microbiological techniques and even when identified would have taken a long time. However, improved sample extraction and regular database upgrades with inclusion of local species should overcome the limitations thus providing a report relevant to the clinician for patient management. VITEK MS is easy to perform even for a relatively inexperienced technician and is a rapid method to identify bacteria. Future developments of the databases to include an expanded number of new species and more robust mass spectra profiles for current species will greatly improve the performance and utility of these automated systems for bacterial identification.
[1]. Garrigos T, Neuwirth C, Chapuis A, Bador J, Amoureux L, Development of a database for the rapid and accurate routine identification of Achromobacter species by matrix-assisted laser desorption/ionization-time-of-flight mass spectrometry (MALDI-TOF MS)Clin Microbiol Infect 2020 S1198-743X(20)30176-72 [Google Scholar]
[2]. Clark AE, Kaleta EJ, Arora A, Wolk DM, Matrix-Assisted laser desorption ionization-time of flight mass spectrometry: A fundamental shift in the routine practice of clinical microbiologyClin Microbiol Rev 2013 26(3):547-603.10.1128/CMR.00072-1223824373 [Google Scholar] [CrossRef] [PubMed]
[3]. Price EP, Arango VS, Kidd TJ, Fraser TA, Nguyen TK, Bell SC, Duplex real-time PCR assay for the simultaneous detection of Achromobacter xylosoxidans and Achromobacter sppMicrob Genomics 2020 6(7):01-11.10.1099/mgen.0.00040632667877 [Google Scholar] [CrossRef] [PubMed]
[4]. Gomila M, Prince-Manzano C, Svensson-Stadler L, Busquets A, Erhard M, Martínez DL, Genotypic and phenotypic applications for the differentiation and species-level identification of Achromobacter for clinical diagnosesPLoS One 2014 9(12):01-22.10.1371/journal.pone.011435625474264 [Google Scholar] [CrossRef] [PubMed]
[5]. Pandey K, Nautiyal S, Achromobacter: An emerging nosocomial pathogenInt J Res Med Sci 2019 7(8):3090-94.10.18203/2320-6012.ijrms20193400 [Google Scholar] [CrossRef]
[6]. Günther F, Merle U, Frank U, Gaida MM, Mutters NT, Pseudobacteremia outbreak of biofilm-forming Achromobacter xylosoxidans- environmental transmissionBMC Infect Dis [Internet] 2016 16(1):01-05.Available from: http://dx.doi.org/10.1186/s12879-016-1909-010.1186/s12879-016-1909-027756240 [Google Scholar] [CrossRef] [PubMed]
[7]. Lee JH, Lee SY, Park IY, Park SY, Lee JS, Kang G, A case of septic shock caused by Achromobacter xylosoxidans in an immunocompetent female patient after extracorporeal shock wave lithotripsy for a ureteral stoneInfect Chemother 2016 48(1):47-50.10.3947/ic.2016.48.1.4727104016 [Google Scholar] [CrossRef] [PubMed]
[8]. Barragán EP, Pérez JS, Corbella L, Orellana MÁ, Fernández-Ruiz M, Achromobacter xylosoxidans bacteremia: Clinical and microbiological features in a 10-year case seriesRev Esp Quimioter 2018 31(3):268-73. [Google Scholar]
[9]. Tripathi N, Koirala N, Kato H, Singh T, Karri K, Thakur K, First documented case of percutaneous endoscopic gastrostomy (PEG) tube-associated bacterial peritonitis due to Achromobacter species with literature reviewCase Rep Gastrointest Med 2020 2020(2):01-11.10.1155/2020/439793032047677 [Google Scholar] [CrossRef] [PubMed]
[10]. Tena D, González-Praetorius A, Pérez-Balsalobre M, Sancho O, Bisquert J, Urinary tract infection due to Achromobacter xylosoxidans: Report of 9 casesScand J Infect Dis 2008 40(2):84-87.10.1080/0036554070155871417852927 [Google Scholar] [CrossRef] [PubMed]
[11]. Suryavanshi KT, Lalwani SK, Uncommon pathogen: Serious manifestation: A rare case of Achromobacter xylosoxidans septic arthritis in immunocompetetant patientIndian J Pathol Microbiol 2015 58(3):395-97.10.4103/0377-4929.16292926275277 [Google Scholar] [CrossRef] [PubMed]
[12]. Veschetti L, Sandri A, Johansen HK, Lleò MM, Malerba G, Hypermutation as an evolutionary mechanism for Achromobacter xylosoxidans in cystic fibrosis lung infectionPathogens 2020 9(2):01-10.10.3390/pathogens902007231973169 [Google Scholar] [CrossRef] [PubMed]
[13]. Di LJ, Cao F, Ding YX, Wu YD, Guo YL, Li F, Timing, distribution, and microbiology of infectious complications after necrotising pancreatitisWorld J Gastroenterol 2019 25(34):5162-73.10.3748/wjg.v25.i34.516231558864 [Google Scholar] [CrossRef] [PubMed]
[14]. Grajales-Figueroa G, Hernández HAD, Portillo MAC, Uscanga LF, Peláez-Luna M, Calleros JH, Increased mortality from extrapancreatic infections in hospitalized patients with acute pancreatitisGastroenterol Res Pract 2019 2019:01-07.10.1155/2019/278976430944558 [Google Scholar] [CrossRef] [PubMed]
[15]. Otta S, Swain B, Panigrahy R, Panda K, Debata NK, Achromobacter xylosoxidans: A rare pathogen for community-acquired acute pancreatitisJMM Case Reports 2014 1(3):03-05.10.1099/jmmcr.0.T00022 [Google Scholar] [CrossRef]
[16]. Weitkamp JH, Tang YW, Haas DW, Midha NK, Crowe JE, Recurrent Achromobacter xylosoxidans bacteremia associated with persistent lymph node infection in a patient with hyper-immunoglobulin M syndromeClin Infect Dis 2000 31(5):1183-87.10.1086/31746111073750 [Google Scholar] [CrossRef] [PubMed]
[17]. Eshwara VK, Mukhopadhyay C, Mohan S, Prakash R, Pai G, Two unique presentations of Achromobacter xylosoxidans infections in clinical settingsJ Infect Dev Ctries 2011 5(2):138-41.10.3855/jidc.125821389595 [Google Scholar] [CrossRef] [PubMed]
[18]. CLSI. Performance standards of antimicrobial susceptibility testing. M100. 30th ed. CLSI supplement M100. Wayne PA. Clinical and Laboratory Standards Institute. 2020 [Google Scholar]
[19]. Crockett SD, Wani S, Gardner TB, Falck-Ytter Y, Barkun AN, Crockett S, American Gastroenterological Association Institute Guideline on Initial Management of Acute PancreatitisGastroenterology 2018 154(4):1096-101.10.1053/j.gastro.2018.01.03229409760 [Google Scholar] [CrossRef] [PubMed]
[20]. Goodchild G, Chouhan M, Johnson GJ, Practical guide to the management of acute pancreatitisFrontline Gastroenterol 2019 10(3):292-99.10.1136/flgastro-2018-10110231288253 [Google Scholar] [CrossRef] [PubMed]
[21]. Yabuuchi E, Oyama A, Achromobacter xylosoxidans new species from human ear dischargeJapan J Microbiol 1971 15(5):477-81.10.1111/j.1348-0421.1971.tb00607.x5316576 [Google Scholar] [CrossRef] [PubMed]
[22]. Steinberg JP, Lutgring JD, Burd EM, Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases 2020 9th edPhiladelphiaElsevierChapter 236, Other Gram-negative and Gram variable bacilli; 2847-2864 [Google Scholar]
[23]. Shahul HA, Manu MK, Mohapatra AK, Chawla K, Chryseobacterium indologenes pneumonia in a patient with non-Hodgkin’s lymphomaBMJ Case Rep 2014 2014:10-11.10.1136/bcr-2014-20459025249217 [Google Scholar] [CrossRef] [PubMed]
[24]. Whistler T, Sangwichian O, Jorakate P, Sawatwong P, Surin U, Piralam B, Identification of gram negative nonfermentative bacteria: How hard can it be?PLoS Negl Trop Dis 2019 13(9):01-16.10.1371/journal.pntd.000772931568511 [Google Scholar] [CrossRef] [PubMed]
[25]. Papalia M, Figueroa-Espinosa R, Steffanowski C, Barberis C, Almuzara M, Barrios R, Expansion and improvement of MALDI-TOF MS databases for accurate identification of Achromobacter speciesJ Microbiol Methods 2020 172:01-04.10.1016/j.mimet.2020.10588932171844 [Google Scholar] [CrossRef] [PubMed]
[26]. Nomura F, Tsuchida S, Murata S, Satoh M, Matsushita K, Mass spectrometry-based microbiological testing for blood stream infectionClin Proteomics [Internet] 2020 17(1):01-11.Available from: https://doi.org/10.1186/s12014-020-09278-710.1186/s12014-020-09278-732435163 [Google Scholar] [CrossRef] [PubMed]