Anxiety disorders have a global impact on the disability adjusted life years, being ranked at sixth position in the global burden of diseases affecting over 250 million people [1]. The morbidity associated with these disorders worsens with time and significantly impairs day-to day activities.
Conventional pharmacological therapy of anxiety disorders includes only certain class of drugs. Selective Serotonin Reuptake Inhibitors (SSRIs) and Serotonin-Norepinephrine Reuptake Inhibitors (SNRIs) are among the first line of therapy [2]. Treatment-refractory anxiety occurs in every one out of three patients diagnosed with anxiety disorders. The major reasons being the risk of abuse, delayed onset of therapeutic action and non-compliance to treatment due to withdrawal symptoms which mimics the anxiety disorder symptoms [3,4]. In recent years, complementary medicines such as herbal remedies are on the rise in the management of anxiety disorders in order to counteract the limitations of the conventional medical therapy [5].
Curcumin, an active phytochemical flavonoid, extracted from the rhizomes of the perennial plant Curcuma longa, commonly known as turmeric, has long since been used in traditional medicine for its potent anti-inflammatory and antioxidant properties in India. It has a diverse mechanism of action. In mouse brain, curcumin has shown to induce the monoamine neurotransmitters serotonin and dopamine [6]. Essentially, serotonin is found to be the most prominent neurotransmitter in modulating the brain state in anxiety [7].
Literature shows that curcumin-metal ion complexes are more efficacious than curcumin when used alone [8-10]. Zinc, a trace element obtained from dietary sources is essential for protein synthesis and cell division. Evidences reveal that zinc plays a key role in human neurodevelopment. And supplementation of zinc enhanced the efficacy of antidepressant drugs through synergistic action [11].
The laboratory mouse is recognised as prominent model for neurobehavioural preclinical tests since it exhibits anxiety-related behaviours [12]. Hence, the aim of the study was to evaluate the synergistic antianxiety effect of curcumin and zinc on acute and chronic models of anxiety in male swiss albino mice.
Materials and Methods
The experimental animal study was conducted in January, 2018 in the animal house at the Sri Manakula Vinayagar Medical College and Hospital. All procedures in the study were reviewed and approved by the Institutional Animal Ethics Committee (IAEC) (IAEC/SMVMCH/023/2017). The care and maintenance of the animals were based on the guidelines of the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA).
Study Subjects
A total of 36 laboratory bred naive healthy male Swiss Albino mice, weighing 20-30 g, were procured from Sri Raghavendra Enterprises, Bangalore. Animals were housed in groups of six in polypropylene cages under standard room temperature (24-27°C) and a 12:12 hours light:dark cycle. The animals were provided with standard pellet diet and water ad libitum. Prior to the experiment, the animals were acclimatised for a period of seven days in animal house. The experiment was conducted during the light period between 10.00 AM and 2.00 PM. All the experimental mice were healthy throughout the study.
Drugs and Drug Administration
Curcumin {1,7-bis(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione}, zinc chloride (anhydrous powder) and peanut oil (arachis oil) were procured from Sigma Aldrich, USA. Diazepam was obtained from Ranbaxy labs, India. The drug solutions were freshly prepared on the day of the administration. Curcumin was administered per oral after dilution with peanut oil to a concentration of 5 and 10 mg/kg. Zinc chloride dissolved in double distilled water was administered intraperitoneally (IP) at dose of 10 mg/kg body weight. The standard anxiolytic drug used in this study was diazepam diluted with sterile saline at a dose of 3 mg/kg IP. The peanut oil was used as control and was administered orally. Each of the drug dosage was selected on the basis of previous studies [13,14].
Experimental Design
The thirty-six mice were randomly assigned to six groups. Each group comprised of six animals to study the acute and chronic anxiolytic effect in anxiety models such as the EPM test and light/dark box test. Each animal was tested initially in the EPM followed by light/dark box test after administration of the drug/vehicle one hour prior to the experiment in acute study. Following a washout period of one week the animals were utilised for the study of chronic anxiolytic effect wherein the drugs were administered once daily for 14 days and the last dose was administered on the 14th day one hour before the experiment.
Experimental grouping of animals:
Group 1-Peanut oil p.o (control)
Group 2-Diazepam 3 mg/kg IP (standard drug)
Group 3-Curcumin 5 mg/kg; p.o
Group 4-Curcumin 10 mg/kg; p.o
Group 5-Curcumin 5 mg/kg p.o + Zinc chloride 10 mg /kg; IP
Group 6-Curcumin 10 mg/kg p.o + Zinc chloride 10 mg/kg IP
The animals were treated in the same chronological order throughout the study period. The animals were coded in different colours and each colour referred each group, respectively.
Apparatus
Elevated Plus Maze (EPM) test
The EPM test is a well-known behavioural test which is based on the natural aversion of rodents to height and open spaces. The model consists of a maze in the shape of plus symbol with two unprotected or open arms (50×10 cm) and two closed or protected arms (50×10 cm), all elevated approximately 50 cm from the floor. Both arms are separated by central square (10 cm). Anxiolytic treatments results in increased exploration of the open arms by the rodents [15].
Light/Dark box test
Like EPM, the light/dark exploration test is used to assess anxiety-related behaviour of mice. This model consists of wooden box measuring about 60×40×35 cm which is divided into two equal compartments by wooden board with a small doorway (10×10 cm) located centrally at the floor level which connects both the compartments. One compartment (protected) is painted black and covered with a wooden lid. The other compartment (unprotected) is covered by a transparent glass, painted white and illuminated by a 60 watts light bulb which is set 30 cm above the box. Anxiolytic treatment increases the number of transitions between the two compartments, without altering the preference of the mice to spend more time in the dark compartment [16].
Experimental Procedure
Each animal was tested initially in the EPM followed by light/dark box test. After administration of drug/vehicle one hour prior to the experiment in acute study and the last dose given 60 minutes prior to the test on the 14th day in case of chronic study, each animal was placed in the central square of the EPM facing with access to any of the arms. The animal was freely allowed to explore the model for five minutes. Behaviour outcomes recorded were the number of entries and the amount of time spent in open and closed arms in the time period of five minutes.
Following EPM, the mice were placed individually in the centre of the illuminated compartment of the light and dark box, facing away from the dark compartment. The animal was freely allowed to explore the model for five minutes while the outcomes recorded were frequency of entries and the time spent in each compartment.
Behavioural changes in each model were video recorded. Further, following the test of each animal, the apparatuses were cleaned with 70% ethanol between the sessions to mask the odour of the animal.
Statistical Analysis
Data were entered in Microsoft excel 2017. Statistical analysis was done using Statistical Package for the Social Sciences (SPSS) software version 24. Results were expressed as Mean±Standard Error of Mean (SEM). The statistical analysis among the groups was evaluated by one-way analysis of variance (ANOVA). Post-hoc differences between group means were done using Fisher’s Least Significance Difference (LSD) test. The p<0.05 was considered statistically significant.
Results
All the experimental mice were healthy at the end of the study and there was no incidence of mortality or changes in the body weight or external physical appearance of mice noted.
Elevated Plus Maze (EPM)
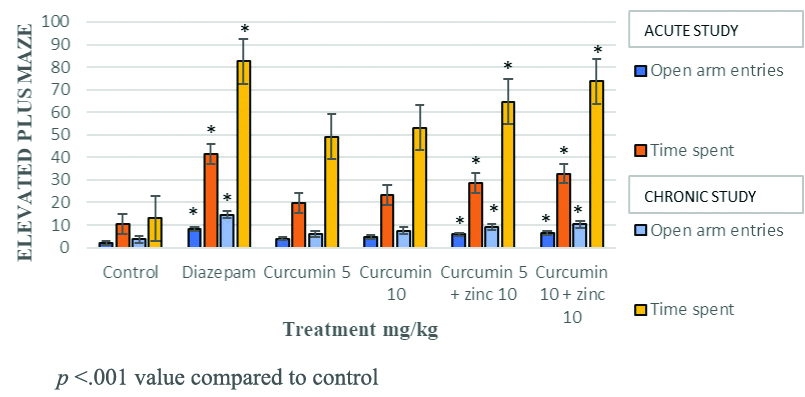
Diazepam significantly increased the number of entries and time spent in open arm and also a reduction in time spent in closed arm. Curcumin at doses of 5 mg/kg and 10 mg/kg with zinc chloride 10 mg/kg showed a significant increase in the number of entries to open arms compared to curcumin when used alone at 5 mg/kg in the acute and chronic models of EPM [Table/Fig-1,2 and 3].
Effect of curcumin-zinc and curcumin on behaviour of mice in Elevated Plus Maze (EPM) - acute study.
| Group (n=6) | Treatment | Number of arm entries | Time spent (seconds) |
|---|
| Open arm | Closed arm | Open arm | Closed arm |
|---|
| 1 | Peanut oil (control) | 2.17±0.47 | 18.17±1.81 | 10.50±0.76 | 289.50±0.76 |
| 2 | Diazepam 3 mg/kg | 8.17±0.60*** | 6.17±0.65*** | 41.50±2.51*** | 258.50±2.51*** |
| 3 | Curcumin 5 mg/kg | 4.0±0.36** | 14.50±0.76** | 19.83±1.81* | 280.17±1.81* |
| 4 | Curcumin 10 mg/kg | 4.50±0.42** | 13.50±0.76** | 23.17±2.22** | 276.83±2.22** |
| 5 | Curcumin 5 mg/kg + Zinc chloride 10 mg/kg | 5.83±0.47***††† | 9.17±0.47***†‡‡ | 28.83±4.81***† | 271.17±4.81***† |
| 6 | Curcumin 10 mg/kg + Zinc chloride 10 mg/kg | 6.67±0.66***†††‡‡ | 9.67±0.88***††‡‡ | 32.83±3.19***††‡ | 265.16±4.34***††‡‡ |
*p<0.05, **p<0.01, ***p<0.001 as compared with control (Group 1); †p<0.05, ††p<0.01, †††p<0.001 as compared with curcumin 5 mg/kg (Group 3); ‡p<0.05, ‡‡p<0.01, ‡‡‡p<0.001 as compared with curcumin 10 mg/kg (Group 4); Values are expressed as Mean±SEM
Effect of curcumin-zinc and curcumin on behaviour of mice in Elevated Plus Maze (EPM) - chronic study.
| Group (n=6) | Treatment | Number of arm entries | Time spent (seconds) |
|---|
| Open arm | Closed arm | Open arm | Closed arm |
|---|
| 1 | Peanut oil (control) | 3.67±0.33 | 34.33±2.49 | 13.0±1.93 | 287±1.93 |
| 2 | Diazepam 3 mg/kg | 14.50±0.92*** | 11.83±1.01*** | 82.50±2.86*** | 217±2.86*** |
| 3 | Curcumin 5 mg/kg | 6.0±0.57* | 28.83±3.43* | 49.17±3.12*** | 250±3.12*** |
| 4 | Curcumin10 mg/kg | 7.50±0.88*** | 25.8±2.30** | 53.17±2.38*** | 246.83±2.38*** |
| 5 | Curcumin 5 mg/kg + Zinc chloride 10 mg /kg | 9.17±0.87***†† | 20.17±1.66***†† | 64.67±2.40***†††‡‡ | 236±3.07***†††‡‡ |
| 6 | Curcumin 10 mg/kg + Zinc chloride 10 mg/kg | 10.33±0.80***††‡‡ | 18.50±1.83***††‡ | 73.83±2.72***†††‡‡‡ | 225±2.82***†††‡‡‡ |
*p<0.05, **p<0.01, ***p<0.001 as compared with control (Group 1); †p<0.05, ††p<0.01, †††p<0.001 as compared with curcumin 5 mg/kg (Group 3); ‡p<0.05, ‡‡p<0.01, ‡‡‡p<0.001 as compared with curcumin 10 mg/kg (Group 4); Values are expressed as Mean±SEM
Effect of curcumin-zinc, curcumin, diazepam on behaviour of mice in the number of entries and time spent in open arm in Elevated Plus Maze (EPM).
Light/Dark Box Test
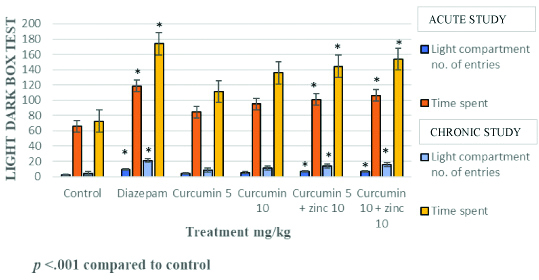
Curcumin at 5 mg/kg and 10 mg/kg with zinc chloride 10 mg/kg showed a significant increase in the number entries to the bright compartment and also increase in the time spent in the bright compartment compared to control group and also curcumin when used alone at 5 mg/kg. Further, the standard drug diazepam (3.0 mg/kg) also significantly increased the number of entries and the time spent in the light compartment in both acute and chronic study [Table/Fig-4,5 and 6].
Effect of curcumin-zinc and curcumin on behaviour of mice in light dark box test- acute study.
| Group (n=6) | Treatment | Number of entries | Time spent (seconds) |
|---|
| Light compartment | Dark compartment | Light compartment | Dark compartment |
|---|
| 1 | Peanut oil (control) | 2.16±0.47 | 24.16±1.85 | 66.0±4.76 | 234.0±4.76 |
| 2 | Diazepam 3 mg/kg | 9.16±0.65*** | 7.50±0.88*** | 118 .50±3.74*** | 181.50±3.74*** |
| 3 | Curcumin 5 mg/kg | 4.16±0.47** | 18.66±2.13* | 84.50±3.59*** | 215.50±3.59*** |
| 4 | Curcumin 10 mg/kg | 5.33±0.49*** | 16.16±1.44*** | 95.16±3.09*** | 204.83±3.09*** |
| 5 | Curcumin 5 mg/kg + Zinc chloride 10 mg/kg | 6.50±0.56***†† | 12.50±1.76***†† | 101.16±3.43***†† | 198.83±3.43***†† |
| 6 | Curcumin 10 mg/kg + Zinc chloride 10 mg/kg | 7.0±0.57***††‡ | 12.16±1.13***††‡ | 106.16±2.72***†††‡ | 193.83±2.71***†††‡ |
*p<0.05, **p<0.01, ***p<0.001 as compared with control (Group 1); †p<0.05, ††p<0.01, †††p<0.001 as compared with curcumin 5 mg/kg (Group 3); ‡p<0.05, ‡‡p<0.01, ‡‡‡p<0.001 as compared with curcumin 10 mg/kg (Group 4); Values are expressed as Mean±SEM
Effect of curcumin-zinc and curcumin on behaviour of mice in light dark box test - chronic study.
| Group | Treatment | Number of entries | Time spent (sec) |
|---|
| Light compartment | Dark compartment | Light compartment | Dark compartment |
|---|
| 1 | Peanut oil (control) | 4.16±0.87 | 44.66±2.33 | 72.50±3.10 | 227.50±3.10 |
| 2 | Diazepam 3 mg/kg | 21.33±1.89*** | 15.16±1.77*** | 173.83±2.67*** | 126.16±2.67*** |
| 3 | Curcumin 5 mg/kg | 8.50±11.16* | 36.50±3.27* | 111.33±3.41*** | 188.66±3.41*** |
| 4 | Curcumin 10 mg/kg | 11.16±1.07*** | 29.83±2.41*** | 135.83±4.42*** | 164.16±4.42*** |
| 5 | Curcumin 5 mg/kg + Zinc chloride 10 mg/kg | 13.66±1.83***†† | 23.0±2.23***†††‡ | 144.5±11.95*** | 155.50±11.95***†† |
| 6 | Curcumin 10 mg/kg + Zinc chloride 10 mg/kg | 15.83±1.35***†††‡ | 22.16±1.97***†††‡ | 153.83±9.28***†‡ | 146.16±9.28***†††‡ |
*p<0.05, **p<0.01, ***p<0.001 as compared with control (Group 1); †p<0.05, ††p<0.01, †††p<0.001 as compared with curcumin 5 mg/kg (Group 3); ‡p<0.05, ‡‡p<0.01, ‡‡‡p<0.001 as compared with curcumin 10 mg/kg (Group 4); Values are expressed as Mean±SEM
Effect of curcumin-zinc, curcumin, diazepam on behaviour of mice in the number of entries and time spent in the light compartment in light dark box test.
Discussion
In the EPM test, the efficacy of curcumin at doses 5 and 10 mg/kg combined with zinc chloride 10 mg/kg revealed significant increase in the number of entries to open arm and the time spent in open arms compared to control group and also to curcumin when used alone at doses of 5 mg/kg and 10 g/kg in both the acute and chronic models. Further, the increase in number of entries and in time spent in open arm by the standard drug diazepam justifies its anxiolytic effects. In the light/dark box test, in acute study the synergistic effect of curcumin and zinc significantly increased the number of entries into the bright compartment and the time spent in the light area compared to the control group. On chronic administration of Curcumin at doses of 5 mg/kg and 10 mg/kg the number of entries and time spent in the bright area are increased and significantly decreased the number of entries into the dark compartment to that of control in a dose-dependent manner.
Study done by Benammi H et al., revealed the antianxiety efficacy of curcumin in lead induced anxiety in wistar rats and the increase in level of serotonin in the dorsal raphe nucleus by immunohistochemistry [17]. Further, the potential antianxiety, antidepressant and antipsychotic-like effects of Zn metal ion in the EPM model of animal in rats was shown by a study done by Joshi M et al., [14].
Diazepam and other benzodiazepine class of drugs are known positive allosteric modulators of the GABA receptor complex [18]. One of the remarkable property of the phytometabolite flavonoids in modulation of GABA receptor complex has re-emerged for in research of its anxiolytic action in the central nervous system [19]. Curcumin is one such flavonoid phytometabolite with significant anxiolytic properties studied in rat models of post-traumatic stress disorder and lead-induced anxiety [17,20].
Zinc metal ion is also proposed to be a potent glutamate N-Methyl-D-Aspartate (NMDA) receptor inhibitor by binding to the GABA receptor. The increased efficacy of anxiolytic drugs on concurrent administration of a lower dose of Zn suggest that Zn could play a beneficial role when used synergistically in anxiety disorders [21].
This study is believed to be the first of its kind to study the synergistic anxiolytic effect of curcumin and zinc. The synergistic action could be attributed to the induction of monoamine neurotransmitter serotonin in modulating the brain state in anxiety.
Limitation(s)
The limitation of the study is that the neurochemical and immuno histochemical tests to evolve the mechanism of the synergistic anxiolytic activity of curcumin and zinc were not included.
Conclusion(s)
The present study demonstrated the synergistic anxiolytic efficacy of curcumin and zinc in acute and chronic models of anxiety in male Swiss Albino Mice. Further studies are required to elucidate the neurochemical basis of its antianxiety effect.