The Cigarette Smoking (CS) is the inhalation of smoke from burned dried leaves of the tobacco plant, mainly in the form of cigarette [1]. Smoking should be considered a pandemic due to citing the death of five million individuals worldwide every year by smoking-related diseases and death [2].
Cigarette smoke is a mixture of more than 4000 different chemicals constituents. Nicotine (3-{1-Methyl-2- pyrrolidinyl} pyridine, is one important alkaloid contained in tobacco leaves. The nicotine is extracted from the dried leaves of the tobacco plant (Nicotinia tabaum and N. rustica). Nicotine is primarily metabolised by the liver, lungs and kidney and half-life of two hours [3]. Cigarette smoke is rich in ROS and RNS, such as nitrogen, alkoxyl and peroxylradicals. These can cause the production of other free radicals, which, in turn, initiate lipid peroxidation on the Low Density Lipoprotein (LDL) particle and cause endothelial cell dysfunction. Smoking may enhance oxidative stress through generation of ROS, thereby causing lipid peroxidation. MDA is an organic compound with the formula CH2 (CHO)2 and is used as a biomarker to measure the level of oxidative stress by a variety of chemical tests and the most frequently used Thiobarbituric (TBA) reaction [4].
ROS generated by compounds containing cigarette smoke, which can directly or indirectly damage DNA, increasing inflammation, thus promoting carcinogenesis in cigarette smokers. In epidemiological studies the Oxidised Guanine/guanosine (OxGua) molecule, 8-hydroxydeoxyguanine (8-OHdG) has been used as biomarkers to assess the intensity of ROS-induced DNA damage [5].
Smoking disturbed the antioxidant enzyme balance. Antioxidant enzymes deactivate free radicals before they attacks cellular components. Antioxidant enzymes act by decreasing the energy of the free radicals or by giving up some of their electrons for its use, thereby causing it to become stable [4].
SOD is metallo-enzyme and is considered the first line of defense because it firstly catalyses in the system harvesting oxygen-free radicals, therefore SOD prevents the oxidation of biological molecules [6]. GPx is a tetra metric enzyme having four 22 KDa monomers, a selenocysteine moiety is also present in the active site of this enzyme. Four subspecies of GPx catalyzes the reduction of hydrogen peroxide and organic hydro peroxides ROOH to water [7]. CAT, a tetra metric enzyme and acts catalytically remove hydrogen peroxides (H2O2) by forming water and oxygen. It is mainly present in the Peroxisomes of mammalian cells [8].
SOD, CAT and GPx are the free radical scavenger enzymes have the ability to inhibit oxidative stress by scavenging the highly destructive free radicals. MDA has a potentially important contribution to DNA damage and mutation, and it has been shown to be mutagenic in bacterial and mammalian cell assays, and it is carcinogenic also. If there is an excessive production of free radicals from exogenous sources added to the endogenous production, the available tissue defense system becomes sluggish resulting in oxidative damage to the tissues and leads to large number of human diseases including ischemic heart disease, cancer, diabetes mellitus, respiratory diseases and ageing [8]. Therefore, the present study was undertaken to assess the extent of lipid peroxidation (also with new marker 8-OHdG) and the status of antioxidants enzymes in cigarette smokers.
Materials and Methods
The present case control study was carried out in the Department of Biochemistry, Government Medical College, Haldwani, and Department of Biochemistry, Santosh Medical College, Ghaziabad during the period of September 2016 to September 2019. Institutional Ethical Clearance has been obtained prior to the study (F. No SU/2018/528 (2).
Inclusion criteria: About 284 healthy cigarette smokers (without any systemic diseases) in the age group of 18-60 years compared with age and sex matched 284 controls (nonsmokers) were included in the study.
Exclusion criteria: Patients with chronic liver diseases, tuberculosis, Pulmonary disorders, Coronary artery diseases, diabetes mellitus, renal failure, thyroid dysfunction, anaemia, malnourished individuals, person with habit of tobacco chewing along with smoking and taking other forms of smoke (bidi, hookah, cigar etc.,) were excluded from the study.
Sample Size Estimation
According to prevalence, used in previous study [9] sample size is calculated
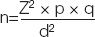
Where, n is the sample size, Z is 1.96 (5% level of significance), p is prevalence, q is 1-p and d is 0.05 (95% of c.f.). According to this formula sample size was 284 for cigarette smokers.
A detailed history from cigarette smokers comprising of number of cigarettes per day and duration of CS was recorded on participant proforma. Subjects were classified into different groups based on number of cigarettes per day and duration of CS i.e., smoking 1-15 cigarette/day <5 years are mild smokers in group I, 15-20 cigarette/day <5 years in group II, 15-20 cigarette/day 5-10 years moderate, and 15-20 cigarette/day >10 years are heavy smokers [10]. Out of total 284 cigarette smokers, 129 were in group I, 42 were in group II which were in mild group, 36 were in moderate and 77 were in heavy group smokers.
All aseptic precautions were taken; with a disposable syringe about five mL of blood was drawn by veinpuncture from a peripheral vein. For the retraction of clot collected blood in clean dry glass tubes was allowed to stand for 30 minutes at room temperature. Then it was centrifuged at 3000 rpm. for ten minutes to obtained the serum. The serum was stored at 4°C in the refrigerator for analysis.
Estimation was done including serum 8-OHdG (8-hydroxyde oxyguanosine) by ELISA (Elabscience, Catalog No. E-EL-0028), MDA by TBARS (Elabscience, Catalog No: E-BC-K025, Superoxide Dismutae (SOD) by water soluble tetrazolium salt 1 (wst-1), GPx (GSH-pX) (Elabscience, Catalog No: E-BC-K096) and CAT by colorimetric method (Elabscience, Catalog No: E-BC-K019).
Statistical Analysis
The analysis was carried out using the SPSS 19.0.2 program for windows. Unpaired t-test and one-way ANOVA were used to analyse all the data for statistical significance.
Results
In present study, out of total 284 cigarette smokers, 272 were males and 12 were females. Mean age of the cigarette smokers and nonsmokers were 40.66±11.08 years and 37.42±9.73 years, respectively. About 12.68% (n=36) of the cases with mild smokers (group I), 0.35% (n=1) (group II), 1.41% (n=4) moderate and 0.70% (n=2) of the cigarette smokers with heavy smokers were in the age group 18-29 years followed by 18.66% (n=53), 1.06% (n=3), 4.23% (12) and 3.16% (n=9) within 30-41 years, 10.92% (n=31), 4.93% (n=14), 1.76 (n=5) and 12.32 (n=35) within 42-53 years and 3.16% (n=9), 8.45 (n=24), 5.28 % (n=15) and 10.92 (n=31) within 54-60 years when divided in to number of cigarette smoked in cigarette smokers.
About 15.14% of the cigarette smokers were in the age group 18-29 years followed by 27.11% within 30-41 years, 29.93% within 42-53 years and 27.82 within 54-60 years [Table/Fig-1].
Distribution of age-group among cigarette smokers (n=284).
| Age group (years) | Male | Female | Total |
|---|
| No | % | No | % | No | % |
|---|
| 18-29 | 41 | 15.07 | 02 | 16.67 | 43 | 15.14 |
| 30-41 | 75 | 27.57 | 02 | 16.67 | 77 | 27.11 |
| 42-53 | 81 | 29.79 | 04 | 33.33 | 85 | 29.93 |
| 54-60 | 75 | 27.57 | 04 | 33.33 | 79 | 27.82 |
| Total | 272 | 95.77 | 12 | 4.23 | 284 | 100 |
About 1-15 cigarettes/day <5 years maximally consumed by the age group between 30-41 years, followed by 15-20 cigarettes/day <5 years by the age group between 54-60 years, followed by 15-20 cigarette/day 5-10 years by the age group between 54-60 years and 15-20 cigarettes/day >10 years by the age group between 42-53 years and maximum number of total cigarette smokers were found to be age group between 42-53 years [Table/Fig-2].
Distribution of age and sex according to duration and number of cigarette smoked in cigarette smokers.
| Age (years) | 1-15 Cigarettes/day <5 years | 15-20 Cigarettes/day <5 years | 15-20 Cigarettes/day 5-10 years | 15-20 Cigarettes/day >10 years | Total |
|---|
| Males | Females | Males | Females | Males | Females | Males | Females |
|---|
| 18-29 | 34 | 2 | 01 | 0 | 04 | 0 | 02 | 0 | 43 |
| 30-41 | 51 | 2 | 03 | 0 | 12 | 0 | 09 | 0 | 77 |
| 42-53 | 31 | 0 | 10 | 04 | 05 | 0 | 35 | 0 | 86 |
| 54-60 | 08 | 01 | 23 | 01 | 14 | 01 | 30 | 01 | 78 |
| Total | 124 | 05 | 37 | 05 | 35 | 01 | 76 | 01 | 284 |
In this study, serum MDA and 8-OHdG levels were significantly elevated in cigarette smokers as compared to nonsmokers. Present study also showed that levels of serum MDA and 8-OHdG levels were significantly increased when adjusted with duration and number of cigarette smoked in cigarette smokers compared to nonsmoker subjects [Table/Fig-3,4].
Mean serum MDA and 8-OHdG in cigarette smokers and nonsmokers.
| Parameters | Nonsmokers (n=284) Mean±SD | Cigarette smokers (n=284)Mean±SD | t-value | p-value |
|---|
| MDA | 3.90±1.03 | 7.47±1.84 | 9.19 | 0.001* |
| 8-OHdG | 40.04±20.14 | 63.41±22.44 | -9.34 | 0.001* |
Test applied- unpaired ‘t’ test; *statistical significant
Shows the mean Serum MDA and 8-OHdG levels were significantly raised in all cigarette smokers as compared to nonsmokers.
| Duration and number of cigarette smoked | | 1-15 Cigarettes/day <5 years | 15-20 Cigarettes/day <5 years | 15-20 Cigarettes/day 5-10 years | 15-20 Cigarettes/day >10 years |
|---|
| Malondialdehyde (MDA) | Mean±SD | 6.76±1.33 | 7.45±1.86 | 8.44±1.10 | 10.77±1.07 |
| Sum of squares | Mean square | F value | p-value |
| Between groups | 18.726 | 897.90 | 5.93 | 0.001* |
| Within groups | 6.24 | 3.21 |
| 8-OHdG | Mean ±SD | 64.23±22.87 | 64.31±24.73 | 60.33±22.73 | 69.20±27.60 |
| Sum of squares | Mean square | F value | p-value |
| Between groups | 383.65 | 127.886 | 3.98 | 0.017* |
| Within groups | 146531.45 | 525.202 |
Test applied- unpaired ‘t’ test; *Statistical significant
The mean serum Gpx, SOD and CAT levels were also significantly decreased in smokers according to duration and number of cigarette smoked as compared to nonsmokers [Table/Fig-5,6].
Mean and ANOVA analysis of serum MDA and 8-OHdG levels according to duration and number of cigarette smoked in cigarette smokers.
| Parameters | Nonsmokers (n=284) Mean±SD | Cigarette smokers (n=284) Mean±SD | t-value | p-value |
|---|
| Gpx | 274.04±68.37 | 62.55±19.97 | -50.03 | 0.025* |
| SOD | 208.56±75.63 | 44.45±16.60 | -11.75 | 0.001* |
| CAT | 127.82±18.68 | 12.92±10.16 | -12.33 | 0.038* |
Test applied- unpaired ‘t’ test; *Statistical significant
Mean and ANOVA analysis of serum Gpx, SOD and CAT levels according to duration and number of cigarette smoked in cigarette smokers.
| Duration and number of cigarette smoked | | 1-15 Cigarettes/day <5 years | 15-20 Cigarettes/day <5 years | 15-20 Cigarettes/day 5-10 years | 15-20 Cigarettes/day >10 years |
|---|
| Gpx | Mean±SD | 71.57±8.56 | 69.98±6.10 | 27.65±13.28 | 30.33±7.01 |
| Sum of squares | Mean square | F value | p-value |
| Between groups | 17159.63 | 5719.78 | 18.91 | 0.028* |
| Within groups | 84388.32 | 302.46 |
| SOD | Mean ±SD | 3.91±1.09 | 3.66±1.08 | 2.76±0.56 | 1.51±0.75 |
| Sum of squares | Mean square | F value | p-value |
| Between groups | 2182.032 | 727.344 | 19.09 | <0.001 |
| Within groups | 10627.052 | 38.090 |
| CAT | Mean ±SD | 16.02±5.21 | 13.92±3.14 | 1.92±0.67 | 1.55±0.92 |
| Sum of squares | Mean square | F value | p-value |
| Between groups | 51.406 | 17.135 | 13.57 | <0.001 |
| Within groups | 352.070 | 1.262 |
Test applied- unpaired ‘t’ test; *Statistical significant
[Table/Fig-4] shows the mean Serum MDA and 8-OHdG levels were significantly raised in cigarette smokers when adjusted with duration and number of cigarette smoked.
[Table/Fig-5] shows the mean Serum Gpx, SOD and CAT levels were significantly decreased in cigarette smokers as compared to nonsmokers.
[Table/Fig-6] shows the mean Serum Gpx, SOD and CAT levels were significantly decreased in cigarette smokers when adjusted with duration and number of cigarette smoked.
Discussion
Smoking prevalence is highest among people aged 30-49 years (37%) and lowest among youth 15-19 and is also relatively low among people aged 60 or older (24%) and there is wide variation in smoking prevalence of males and females as higher in males in most countries about 60% [11]. In present study, out of total 284 cigarette smokers, 272 were males and 12 were females. In present study, prevalence of smoking is highest for people aged 42 53 years (29.93%) and is also relatively high among people aged 54-60-year-old (27.82%) and lowest among youth 18-29 years (15.14%). Which is also supported by Rani M et al., [12]. On the contrary, Narayan KM et al., in Delhi reported a high proportion of cigarette use as compared to bidi in their studies [13].
Researchers showed that Prevalence of smoking is highest for people aged 42-53 years (37%) and lowest among youth 18-30 years, which supports present study and is relatively low among people aged 60 or older (24%) not supported by present study [14]. Among all age groups men prevalence is more likely to smoke than women. In 2014, 20% of men aged 16 were over smoked compared with 17% of women. In this study, men prevalence is also higher (95.77%) than women (4.23%). Smoking prevalence is highest among young adults, 23% of those aged 16-24 and 24% among the 25-34 age groups [15].
In present study, serum MDA and 8-OHdG levels were significantly elevated in cigarette smokers as compared to nonsmokers. This study also showed that levels of serum MDA and 8-OHdG levels were significantly increased when adjusted with duration and number of cigarette smoked in cigarette smokers compared to nonsmoker subjects.
Kashinakunti SV et al., shows level of MDA was significantly increased in smokers as compared to controls. Results of present study are supports to other authors who found increased lipid peroxidation in smokers [16].
Two-fold increase in TBARS level in mild and four-fold increase in heavy cigarette smokers as compared to those control subjects reported by Jain S et al., [17]. Nielsen F et al., also reported that daily exposure to cigarette smoke correlated with MDA levels in plasma [18]. Craig WY et al., reported no correlation between lipid peroxidation and cigarette smoke exposure in healthy subjects which is not supported by findings of the present study [19].
A meta-analysis of active smoking and cancer, including five studies of lung cancer, one of oral cancer and one of bladder cancer in which bulky DNA adducts and increased level of 8-OHdG were measured a significant association between bulky DNA adducts and cancer in current smokers, while no association was observed in ex- and never smokers found by them [20].
The present study showed that mean serum Gpx, SOD and CAT level were significantly decreased in cigarette smokers as compared to nonsmokers. The mean serum Gpx, SOD and CAT levels were also significantly decreased in smokers according to duration and number of cigarette smoked as compared to nonsmokers. These finding suggesting that there is a mitochondrial dysfunction and subsequent imbalance between releasing of ROS and RNS or chlorine species and synthesis of defensive antioxidant capacity systems from nuclear DNA, resulting in oxidative stress.
The present study observed a decrease in the enzymatic activity in smokers than nonsmokers which might have been caused by higher levels of hydrogen peroxide formation. Similar results were also found by Hou SM et al., found that removal of initial rate of hydrogen peroxide is directly proportional to its concentration [21].
Abou-Seif MAM found that erythrocyte SOD and catalase activities were elevated in smokers and also observed lower plasma vitamin E levels and higher erythrocyte SOD and catalase activities in cigarette smokers in comparison with nonsmokers [22]. These results are inconsistent with the present study data obtained from cigarette smokers. However, another study pointed out increased production of oxygen radical species, and decreases in antioxidant activity were observed in cigarette smokers versus those from nonsmokers [23]. According to the results of Durak I et al., smoking did not affect activities of SOD, catalase and GSH-Px in erythrocytes whereas plasma TBARS increased significantly in smokers. Additionally, Durak I et al., indicated that smoking caused no impairment in the enzymatic antioxidant defense system, because erythrocytes have a potent defense capacity [24].
Zhou JF et al., found lower plasma antioxidant concentrations and elevated plasma and erythrocyte lipoperoxide levels in smokers with longer smoking duration. The same researchers indicated that the SOD, catalase and GSH-Px activities of erythrocytes were significantly lower in smokers than in nonsmokers [25]. Bellizzi MC et al., determined that red blood cells from smokers contained less antioxidant enzymes than those from nonsmokers, despite plasma levels of antioxidants being similar in smokers and nonsmokers [26]. Bingol NK et al., suggested that chronic smoking causes peroxidation reactions in both plasma and erythrocytes [27].
Diken H et al., showed that the mean levels of SOD activity in blood of heavy smokers were lower than those in light smokers [28]. In agreement with the result of this study, A study reported an increased SOD activity in long term smokers compared with short term smokers [29].
These studies are in agreement with present findings that duration of smoking significantly affected the Gpx, SOD, CAT, 8-OHdG and MDA in smoking subjects, as Gpx, CAT and SOD were decreased, MDA and 8OHdG were increased. However, it may not be completely out of place to hypothesise that other factors (like nutrition) could have exacerbated this outcome.
Limitation(s)
The study was limited to the population residing in and around Haldwani and their involvement in the study especially in case of females. Lack of fund, time and manpower prevented the inclusion of a large study group and other sensitive biochemical markers of cigarette smoke.
Conclusion(s)
In conclusion, based on the findings and the other data in the study, It can be speculated that these antioxidant and oxidative stress biomarkers might be useful biomarkers for the selection of smokers with a high risk of developing smoke induced inflammation in pulmonary and cardiovascular diseases and will help to clinicians to formulate novel treating protocol and follow-up for their patients. Frequent assessment these markers especially 8-OHdG by various analytical techniques in blood cells or in urine have established it as a very important biomarker not only for carcinogenesis but also for aging and degenerative diseases should be done, as well as intake of balanced diet and antioxidant supplementation is recommended in smokers. Further, discontinuation of smoking and general awareness needs to be created to minimise the risk of smoke related diseases.
Future cross-sectional, longitudinal and mechanistic studies are needed to determine how CS, oxidative stress and antioxidant enzymes are useful in large populations of cigarette smokers with the inclusion of clinically relevant endpoints are needed to extend these findings.
Test applied- unpaired ‘t’ test; *statistical significant
Test applied- unpaired ‘t’ test; *Statistical significant
Test applied- unpaired ‘t’ test; *Statistical significant
Test applied- unpaired ‘t’ test; *Statistical significant