Prevalence of Para-Aortic Lymphadenopathy in Locally Advanced Cervical Cancer using Computed Tomography: A Retrospective Study
Garba Haruna Yunusa1, Usman Malami Aliyu2
1 Consultant Radiologist and Nuclear Medicine Physician, Department of Radiology, College of Health Sciences, Usmanu Danfodiyo University, Sokoto, Nigeria.
2 Consultant Radiation and Clinical Oncologist, Department of Radiotherapy and Oncology, Usmanu Danfodiyo University Teaching Hospital, Sokoto, Nigeria.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Garba Haruna Yunusa, Department of Radiology, College of Health Sciences, Usmanu Danfodiyo University, Sokoto, Nigeria.
E-mail: garusa2001@gmail.com
Introduction
Determination of status of Para-Aortic Lymph Node (PALN) in patients with cervical cancer is one of the most important factors guiding the treatment. It is essential in determining individualised therapy and prognosis.
Aim
To assess the prevalence of para-aortic lymphadenopathy among patients with advanced cervical carcinoma referred for radiotherapy.
Materials and Methods
A retrospective study of patients referred to Radiotherapy and Oncology Department of Usmanu Danfodiyo University Teaching Hospital, Sokoto, Nigeria was carried out. Patient demographics, history of co-morbid conditions, histology, stage at diagnosis, and Computed Tomographic (CT) scan findings of enlarged PALN greater or equal to 10 mm in short axis diameter were retrieved. Data was analysed using IBM SPSS statistics version 23.
Results
A total of 220 patients, age range 27-84 years with mean age 49.95±11.96 years were studied. The histological diagnosis were squamous cell carcinoma in 182 (87.2%), adenocarcinoma in 22 (10%), clear cell carcinoma in 9 (4.1%) while 7 (3.2%) patients had other histological variants. Co-morbities found were chronic cervicitis and HIV in 27 (12.3%) and 19 (8.6%) of the patients, respectively. CT prevalence of PALN was 23 (10.5%). The prevalence of PALN according to International Federation of Gynaecology and Obstetrics (FIGO) stage were 4.3%, 17.4%, 34.8%, 30.4% and 13.0% in stages IIB, IIIA, IIIB, IVA and IVB, respectively. Clinical stage determined based on physical examination and imaging findings, was shown to be related to PALN by univariate analysis (χ2=29.162, p=0.0001).
Conclusion
This study found a 10.5% prevalence of para-aortic lymphadenopathy and a significant relationship between clinical stage and PALN. This should be taken into consideration during treatment planning for patients with advanced cervical carcinoma.
Clinical stage, Para-aortic lymph nodes, Resource-limited setting, Treatment planning
Introduction
Determination of Para-Aortic Lymph Node (PALN) status in patients with carcinoma of the cervix is one of the most important factors guiding treatment approach. Therefore, assessment of PALN status is essential in determining individualised therapy and prognosis [1,2]. Lymph nodes greater than 10 mm in short axis diameter are considered abnormal while the suggested upper limits for internal, common and external iliac nodes are 7 mm, 9 mm and 10 mm, respectively [3,4]. At least three lymphatic routes lead to cervical cancer spread to the common iliac nodes, from where the tumour can involve the para-aortic nodes [4]. Using a cut-off of >10 mm in short axis diameter on CT as a definition for a PALN metastasis the reported accuracy, sensitivity and specificity were 90-94%, 67-80% and 92-100%, respectively, as confirmed by histological findings [5-7]. Generally, raising the threshold of lymph node size has been reported to increase the specificity and positive predictive value and reduce the sensitivity and negative predictive value. For example, a study reported that a threshold of 15 mm was associated with positive predictive value of 75% on CT [8].
The objectives of this study were to assess the CT prevalence of para-aortic lymphadenopathy, and determine the relationship between PALN and clinical stage among patients in the locality who presented with advanced cervical carcinoma and are referred for radiotherapy.
Materials and Methods
A retrospective study of patients referred to Radiotherapy and Oncology Department of the hospital, from December 2014 to November 2019 was carried out. All cases that fulfilled inclusion criteria within the stated period were taken. Patient demographics, history of co-morbid conditions, histology reports, clinical stage at diagnosis, and CT scan findings were retrieved from the database of Radiation and Oncology Department of the hospital. Patients with incomplete medical records and those without pretreatment CT scan imaging were excluded from the study. A cut-off of 10 mm in short axis diameter was used to determine PALN enlargement on the CT images [3,4]. The study was carried out following approval by the Health Research and Ethical Committee of the hospital.
Statistical Analysis
Data was analysed using IBM SPSS statistics version 23. Independent variables were determined by using Chi-square test. The level of significance was set at p<0.05.
Results
A total of 220 patients, age range 27-84 years with mean 49.95±11.96 years were found eligible for inclusion in the study. The histological diagnosis were squamous cell carcinoma in 182 (82.7%) and adenocarcinoma in 22 (10%) of the cases, clear cell carcinoma in 9 (4.1%), while 7 (3.2%) patients had other histological variants. Co-morbities found were chronic cervicitis and HIV in 27 (12.3%) and 19 (8.6%) of the patients, respectively [Table/Fig-1]. CT prevalence of PALN was 23 (10.5%) [Table/Fig-2,3 and 4]. Prevalence of PALN according to FIGO staging for the 23 patients were 4.3%, 17.4%, 34.8%, 30.4% and 13.0% in stages IIB, IIIA, IIIB, IVA and IVB, respectively [Table/Fig-5]. Of the 23 patients with CT positive PALN, 19 (82.6%) had a histological diagnosis of squamous cell carcinoma. Three (13.0%) out of the 23 patients had HIV as co-morbid infective/inflammatory condition associated with lymphadenopathy. Clinical stage was shown to be related to PALN by univariate analysis (χ2=29.162, p<0.0001). There was no statistically significant difference in the prevalance of PALN among age groups (χ2=9.340, p=0.155), histology (χ2=3.271, p=0.352) and HIV status (χ2=0.632, p=0.427).
Univariate analysis of patients’ demographic and clinical characteristics.
| Variable | Number (%) | PALN, n (%) | χ2 | p-value |
|---|
| Age (years) | | | 9.340 | 0.155 |
| <50 | 105 (47.7) | 10 (43.5) | | |
| ≥50 | 115 (52.3) | 13 (56.5) | | |
| Stage | | | 29.162 | 0.001* |
| I-II | 96 (43.6) | 1 (4.3) | | |
| III-IV | 124 (56.4) | 22 (95.7) | | |
| Histology | | | 3.271 | 0.352 |
| Squamous cell carcinoma | 182 (82.7) | 19 (82.6) | | |
| Adenocarcinoma | 22 (10.0) | 4 (17.4) | | |
| Others | 16 (7.3) | 0 (0) | | |
| HIV status | | | 0.632 | 0.427 |
| Negative | 201 (91.4) | 20 (87) | | |
| Positive | 19 (8.6) | 3 (13) | | |
| Chronic cervicitis | | | 3.593 | 0.058 |
| Yes | 27 (12.3) | 0 (0) | | |
| No | 193 (87.7) | 23 (100) | | |
*Statistically significant; PALN: Para-aortic lymph node
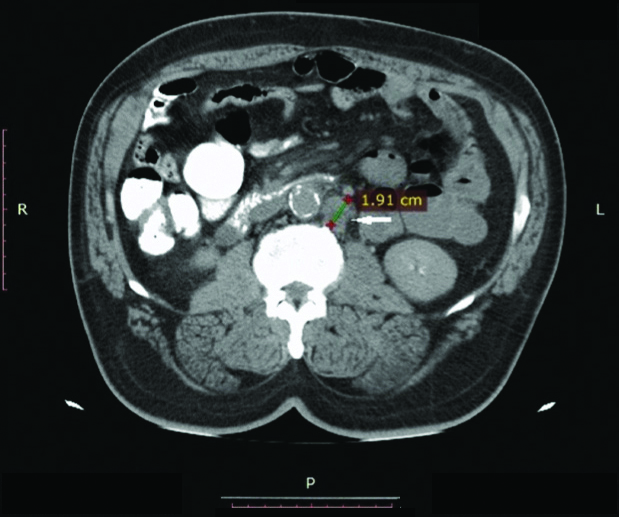
Computed tomography axial image showing 19 mm Para-Aortic Lymph Node (PALN) (arrow) in a 46-year-old female with stage IIIC squamous cell carcinoma of the cervix.
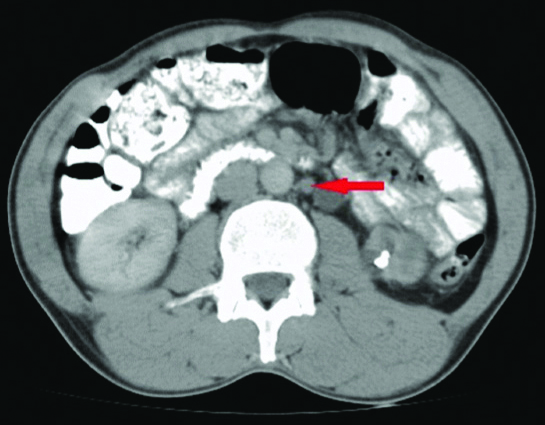
Computed tomography axial image done with oral contrast showing a Para-Aortic Lymph Node (PALN) in a patient with stage IIB adenocarcinoma of the cervix.
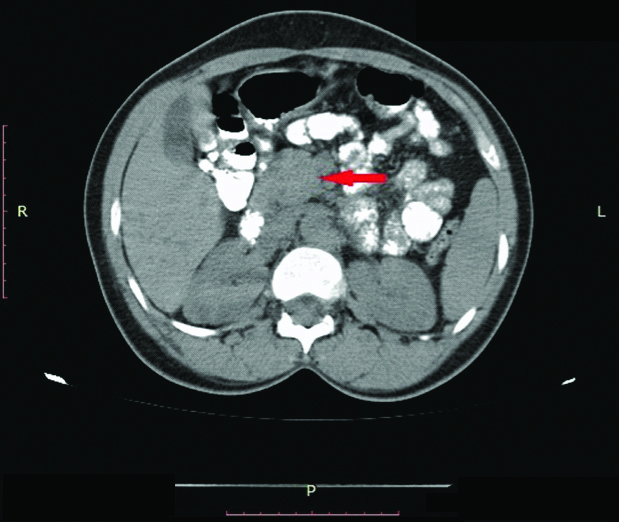
CT of the abdomen showing matted Para-Aortic Lymph Node (PALN) (arrow) in a patient with squamous cell carcinoma of the cervic stage IVB.
Distribution of para-aortic lymph nodes by FIGO stage and age group.
| Age (Years) | FIGO Stage | Total, n (%) | PALN, n (%) |
|---|
| IB | IIA | IIB | IIIA | IIIB&C | IVA | IVB |
|---|
| 20-29 | 0 | 0 | 2 | 6 | 1 | 0 | 0 | 9 (4.1) | 0 (0) |
| 30-39 | 6 | 4 | 5 | 6 | 4 | 1 | 1 | 27 (12.3) | 1 (4.3) |
| 40-49 | 4 | 14 | 19 | 14 | 10 | 5 | 3 | 69 (31.4) | 9 (39.1) |
| 50-59 | 1 | 7 | 8 | 9 | 17 | 7 | 5 | 54 (24.5) | 10 (43.5) |
| 60-69 | 0 | 5 | 16 | 9 | 9 | 5 | 2 | 46 (20.9) | 3 (13.0) |
| 70-79 | 0 | 1 | 1 | 5 | 3 | 1 | 0 | 11 (5.0) | 0 (0) |
| 80-89 | 0 | 3 | 0 | 0 | 1 | 0 | 0 | 4 (1.8) | 0 (0) |
| Total | 11 | 34 | 51 | 49 | 45 | 19 | 11 | 220 (100) | 23 (100) |
| PALN, n (%) | 0 (0) | 0 (0) | 1 (4.3) | 4 (17.4) | 8 (34.8) | 7 (30.4) | 3 (13.0) | | |
*FIGO: International federation of gynaecology and obstetrics; PALN: Para-aortic lymph node
Discussion
A CT finding of PALN involvement in patients with cervical carcinoma is an indication for more aggressive treatment as these groups of patients are considered to be potentially curable. A study by Wu SY et al., conducted to identify prognostic factors in patients with cervical cancer, reported a five-year overall survival rate of 50% in patients with CT-detected PALN metastases following treatment with external beam radiation therapy to metastatic PALNs with concurrent platinum-based chemotherapy [9].
In the current study, squamous cell carcinoma turned out to be the commonest histological type with a prevalence of 87.1%, a finding that is consistent with a similar study by Han X et al., who reported a prevalence of 88.4% [10]. The prevalence of PALN in the current study was 10.5% which is similar to the finding from a study by Tan K and Yao D, who reported a prevalence of 9.9% in a study that was carried out to investigate the incidence and risk factors of PALN metastases in stages IB, IIA and IIB cervical carcinoma [11]. The incidence of PALN varies widely as documented in previous studies ranging from 1 to 11.1% in stage I and from 7.2% to 25% in stage II [10-13]. However, the findings in the index study are 0% and 4.3% for stages I and II respectively, while positive PALN rates for stages IIIA, IIIB, IVA and IVB were 17.4%, 34.8%, 30.4% and 13.0%, respectively. The lower figures in the current study may be due to smaller sample size but nevertheless the increasing trend suggests that patients with more advanced FIGO stage were more likely to have PALN metastases [10,14].
This study found statistically significant relationship between PALN and clinical stage which is consistent with the fact that higher clinical stages are more likely to present with positive PALN. While this finding is consistent with findings from some studies [10,11,15], it differs from that by Sakuragi N et al., who found no relationship between clinical stage and PALN [16]. This difference could be attributed to elements of subjectivity in the clinical staging system. On the other hand, the index study found no significant relationship between PALN and age, histology or HIV status. A larger sample size is suggested to ascertain the validity or otherwise of this finding.
Imaging modalities that have been in use in the assessment of PALN involvement before treatment were Ultrasonography (US), Computed Tomography (CT) and Magnetic Resonance Imaging (MRI). These imaging modalities have produced lower sensitivity, specificity and accuracy in the detection of PALN when compared with Positron Emission Tomography-CT (PET-CT) [2] which is a hybrid imaging modality that combines functional/metabolic and morphologic imaging at the same time. This imaging modality is not available in resource poor settings like ours, hence the emphasis on CT detection of lymph node involvement among the patients was studied. Choi HJ et al., and Wu C et al., conducted meta-analyses and compared the diagnostic performance of CT, MRI and PET or PET-CT in detection of metastatic lymph node status in patients with cervical cancer. They concluded that PET or PET/CT had an overall higher diagnostic performance than CT or MRI in detecting metastatic lymph nodes in patients with cervical cancer [17,18].
Although CT is relatively readily available, it is worthy of note that one of its limitations is that enlarged malignant and hyperplastic nodes cannot be distinguished [5]. Therefore, it is important to note that patients with cervical cancer can have secondary infection or co-morbid infective/inflammatory condition that results in adenopathy [19]. Index study found that 13% of patients with CT detected PALN also had HIV which means only histology would determine the presence of metastases in this group of patients. On the other hand, tumour may be present in normal-sized lymph nodes in a setting of micrometastasis, giving CT a sensitivity of 44% for malignancy [1]. Yang WT et al., reported that the presence of central necrosis in a lymph node increases the CT positive predictive value for malignancy to 100% [20].
Limitation(s)
Some of the limitations of this study include; a retrospective study design, lack of histological confirmation of the lymph node metastases and the inherent lower sensitivity of CT imaging evaluation of PALN compared to PET/CT.
Conclusion(s)
The prevalence of PALN in this study is 10.5% and there is significant relationship between clinical stage and PALN. This should be taken into cognisance during treatment planning for patients with advanced cervical cancer. This finding further underscores the significance of utilisation of abdomino-pelvic CT imaging in the evaluation of PALN among patients with carcinoma of the cervix.
Authors’ contributions: GHY was responsible for concept design of the study, analysis and interpretation of data and drafting the article. UMA was responsible for acquisition of data, interpretation of data and revising the article critically for important intellectual content. All authors approved the final manuscript.
*Statistically significant; PALN: Para-aortic lymph node
*FIGO: International federation of gynaecology and obstetrics; PALN: Para-aortic lymph node
Author Declaration:
Financial or Other Competing Interests: None
Was Ethics Committee Approval obtained for this study? Yes
Was informed consent obtained from the subjects involved in the study? Yes
For any images presented appropriate consent has been obtained from the subjects. Yes
Plagiarism Checking Methods: [Jain H et al.]
Plagiarism X-checker: Apr 15, 2020
Manual Googling: Jun 29, 2020
iThenticate Software: Sep 26, 2020 (19%)
[1]. Jang H, Chun M, Cho O, Heo JS, Ryu HS, Chang SJ, Prognostic factors and treatment outcome after radiotherapy in cervical cancer patients with isolated para-aortic lymph node metastasesJ Gyneol Oncol 2013 24:229-35.10.3802/jgo.2013.24.3.22923875072 [Google Scholar] [CrossRef] [PubMed]
[2]. Gouy S, Morice P, Narducci F, Uzan C, Martinez A, Rey A, Prospective multicenter study evaluating the survival of patients with locally advanced cervical cancer undergoing laprascopic para-aortic lymphadenectomy before chemoradiotherapy in the era of positron emission tomography imagingJ Clin Oncol 2013 31:3026-33.10.1200/JCO.2012.47.352023857967 [Google Scholar] [CrossRef] [PubMed]
[3]. Vinnicombe SJ, Norman AR, Nicolson V, Husband JE, Normal pelvic lymphnodes; evaluation with CT after bipedal lymphangiographyRadiology 1995 194:349-55.10.1148/radiology.194.2.78247097824709 [Google Scholar] [CrossRef] [PubMed]
[4]. Park JM, Charnsangavej C, Yoshimitsu K, Herron DH, Robinson TJ, Wallace S, Pathways of nodal metastasis from pelvic tumours: CT demonstrationRadioGraphics 1994 14:1309-21.10.1148/radiographics.14.6.78553437855343 [Google Scholar] [CrossRef] [PubMed]
[5]. Matsukuma K, Tsukamoto N, Matsuyama T, Ono M, Nakano H, Preoperative CT study of lymph nodes in cervical cancer-its correlation with histological findingsGynecol Oncol 1989 33:16817110.1016/0090-8258(89)90544-1 [Google Scholar] [CrossRef]
[6]. Hwang L, Bailey A, Lea J, Albuquerque K, Para-aortic nodal metastases in cervical cancer: a blind spot in the International Federation of gynecology and Obstetrics staging system: Current diagnosis and managementFuture Oncol 2015 11(2):309-22.10.2217/fon.14.20025591841 [Google Scholar] [CrossRef] [PubMed]
[7]. Camilien L, Gordon D, Fruchter RG, Maiman M, Boyce JG, Predictive value of computerized tomography in the presurgical evaluation of primary carcinoma of the cervixGynecol Oncol 1988 30:209-15.10.1016/0090-8258(88)90026-1 [Google Scholar] [CrossRef]
[8]. Bandy LC, Clarke-Pearson DL, Silverman PM, Creasman WT, Computed tomography in evaluation of extrapelvic lymphadenopathy in carcinoma of the cervixObstet Gynecol 1985 65:73-76. [Google Scholar]
[9]. Wu SY, Huang EY, Chanchien CC, Lin H, Wang CJ, Sun LM, Prognostic factors associated with radiotherapy for cervical cancer with computed tomography-detected para-aortic lymph node metastasisJ Radiat Res 2014 55:129-38.10.1093/jrr/rrt08623814113 [Google Scholar] [CrossRef] [PubMed]
[10]. Han X, Wen H, Ju X, Chen X, Ke G, Zhou Y, Predictive factors of para-aortic lymph nodes metastasis in cervical cancer patients: A retrospective analysis based on 723 para-aortic lymphadenectomy casesOncotarget 2017 8(31):51840-47.10.18632/oncotarget.1602528881693 [Google Scholar] [CrossRef] [PubMed]
[11]. Tan K, Yao D, Risk factors of para-aortic lymph node metastasis in stages IB, IIA and IIB cervical carcinomaInt J Clin Exp Med 2016 9(8):15336-44. [Google Scholar]
[12]. Leblanc E, Narducci F, Frumovitz M, Lesoin A, Castelain B, Baranzelli MC, Therapeutic value of pretherapeutic extraperitoneal laparoscopic staging of locally advanced cervical carcinomaGynecol Oncol 2007 105:304-11.10.1016/j.ygyno.2006.12.01217258799 [Google Scholar] [CrossRef] [PubMed]
[13]. Malzoni M, Tinelli R, Cosentino F, Perone C, Iuzzolino D, Rasile M, Laparoscopic radical hysterectomy with lymphadenectomy in patients with early cervical cancer: our instruments and techniqueSurg Oncol 2009 18:289-97.10.1016/j.suronc.2008.07.00918805001 [Google Scholar] [CrossRef] [PubMed]
[14]. Grigsby PW, Siegel BA, Dehdashti F, Lymph node staging by positron emission tomography in patients with carcinoma of the cervixJ Clin Oncol 2001 19:3745-49.10.1200/JCO.2001.19.17.374511533097 [Google Scholar] [CrossRef] [PubMed]
[15]. Huang H, Liu J, Li Y, Wan T, Feng Y, Li Z, Metastasis to deep obturator and para-aortic lymph nodes in 649 patients with cervical carcinomaEur J Surg Oncol 2011 37:97898310.1016/j.ejso.2011.08.12821907530 [Google Scholar] [CrossRef] [PubMed]
[16]. Sakuragi N, Satoh C, Takeda N, Hareyama H, Takeda M, Yamamoto R, Incidence and distribution pattern of pelvic and paraaortic lymph node metastasis in patients with Stages IB, IIA, and IIB cervical carcinoma treated with radical hysterectomyCancer 1999 85:1547-54.10.1002/(SICI)1097-0142(19990401)85:7<1547::AID-CNCR16>3.0.CO;2-2 [Google Scholar] [CrossRef]
[17]. Choi HJ, Ju W, Myung SK, Kim Y, Diagnostic performance of computer tomography, magnetic resonance imaging, and positron emission tomography or positron emission tomography/computer tomography for detection of metastatic lymph nodes in patients with cervical cancer: Meta-analysisCancer Science 2010 101:1471-79.10.1111/j.1349-7006.2010.01532.x20298252 [Google Scholar] [CrossRef] [PubMed]
[18]. Wu C, Lu L, Liu Y, Mi Y, Diao W, Evaluating MRI, CT, PET/CT in detection of lymph node status in cervical cancer: A meta-analysisInt J Clin Exp Med 2016 9(6):9917-31. [Google Scholar]
[19]. Lewis E, The use and abuse of imaging in gynaecologic cancerCancer 1987 60:1993-2009.10.1002/1097-0142(19901015)60:8+<1993::AID-CNCR2820601511>3.0.CO;2-V [Google Scholar] [CrossRef]
[20]. Yang WT, Lam WWM, Yu MY, Cheung TH, Metreweli C, Comparison of dynamic helical CT and dynamic MR imaging in the evaluation of pelvic lymph nodes in cervical carcinomaAJR Am J Roentgenol 2000 175:759-66.10.2214/ajr.175.3.175075910954463 [Google Scholar] [CrossRef] [PubMed]