A Case of Small Cell Carcinoma of Lung Presenting as Opaque Hemithorax
Keerthan Ganapathi1, Saood Ali2, Ulhas Jadhav3, Babaji Ghewade4
1 Postgraduate Student, Department of Respiratory Medicine, Datta Meghe Institute of Medical Sciences, Wardha, Maharashtra, India.
2 Assistant Professor, Department of Respiratory Medicine, Datta Meghe Institute of Medical Sciences, Wardha, Maharashtra, India.
3 Professor and Head, Department of Respiratory Medicine, Datta Meghe Institute of Medical Sciences, Wardha, Maharashtra, India.
4 Professor, Department of Respiratory Medicine, Datta Meghe Institute of Medical Sciences, Wardha, Maharashtra, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Keerthan Ganapathi, Postgraduate Student, Department of Respiratory Medicine, Datta Meghe Institute of Medical Sciences, Sawangi, Wardha, Maharashtra, India.
E-mail: keerthan.ganapathi@gmail.com
The most common cause of an Opaque Hemithorax is Pleural Effusion. It is a rare occurrence to find patients with extensive mass leading to an opaque hemithorax. Thorough search of literature did not yield any case with such a large lung mass leading to opaque hemithorax, without the presence of pleural effusion. Lung cancer is the most common cancer diagnosed worldwide and has predominantly been attributed to tobacco smoke exposure. Of the several types, small cell lung cancer differs from others by its early spread and extensive dissemination leading to metastatic classification at the time of diagnosis. Here, is a case of a 50-year-old female patient who presented to the outpatient department with dry cough, weight loss and appetite loss since five months. Her chest radiograph revealed a right-sided homogeneous opacity involving the entire right hemithorax and left-sided pleural-based homogenous mass. Contrast Enhanced Computed Tomography (CECT) thorax revealed an enlarged hemithorax with evidence of large, ill-defined heterogeneously enhancing multilobulated soft-tissue density mass lesion occupying the entire right hemithorax. Biopsy was suggestive of Small Cell Carcinoma of Lung (SCLC), chemotherapy was given and patient is under regular follow-up.
Chemotherapy, Lung malignancy, Ultrasound guided biopsy, White out lung
Case Report
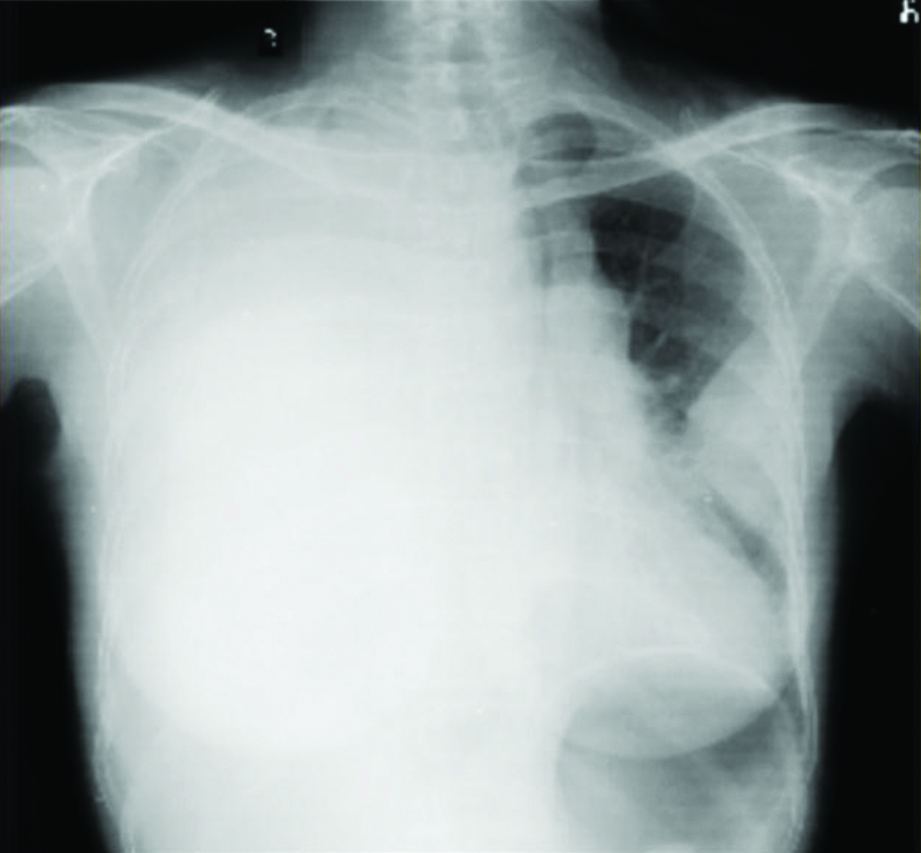
A 50-year-old female patient presented to the outpatient department with complaints of dry cough, weight loss (around 10 kg) and appetite loss, since five months. She had no significant medical history apart from history of biomass exposure for more than 20 years. On examination, she was found to be underweight (BMI 17.31 kg/m2), tachycardiac (110 beats/minute) with baseline saturation of 95% on room air. She had pallor with presence of dilated veins over the neck, anterior chest wall and upper abdomen region, suggestive of Superior Vena Cava Obstruction. Chest examination revealed bulging in the right side, with decreased movement in the right hemithorax and generalised dull note on percussion on the right side and decreased breath sounds on auscultation. Her routine blood investigation revealed anaemia (10.8 g/dL) and rest of the renal and liver function tests were within normal limits. Her chest radiograph [Table/Fig-1] revealed a right-sided homogeneous opacity involving the entire right hemithorax resulting in pushing the mediastinum to the left side and left-sided pleural-based homogenous mass.
Chest radiograph- Posterioanterior (PA) View: Right-sided homogeneous opacity involving the entire right hemithorax resulting in pushing the mediastinum to the left side and left-sided pleural-based lesion.
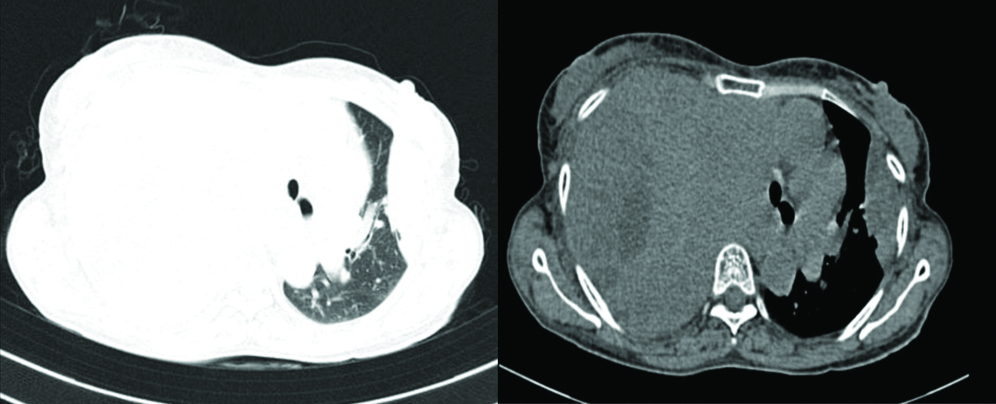
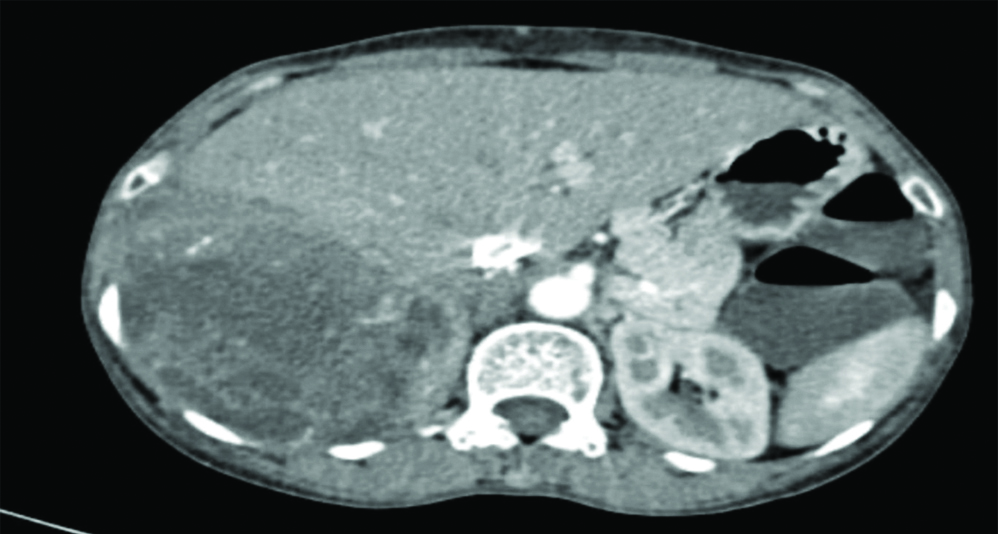
CECT thorax [Table/Fig-2] and abdomen revealed an enlarged hemithorax with evidence of large, ill-defined heterogeneously enhancing multilobulated soft-tissue density mass lesion occupying the entire right hemithorax with non-enhancing hypodense necrotic areas within. Lesion was found to be approximately 38.5×26.2×16.2 cm crossing the midline, pushing the mediastinum and trachea towards the left side with loss of fat plane with adjacent intercostal plane and abutting the right pulmonary artery and arch of aorta. The lesion was found to be displacing the liver and right kidney inferiorly and compressing over the rest of the right lung parenchyma. A similar heterogeneously enhancing lentiform shaped, pleural-based lesion was noted in the left lateral wall of the left hemithorax of approximate size 8.2×5.5×2.7 cm with adjacent fibrotic strands and interlobar septal thickening. Abdominal cuts showed few, small, heterogeneously enhancing hypodense lesions in the liver (largest 5×5 mm), suggestive of hepatic metastasis [Table/Fig-3]. Rest of the abdomen appeared normal.
Cut section of CECT Thorax revealing large ill-defined heterogeneously enhancing mass on the right side with pleural-based lesion on the left side.
Cut section of CT Abdomen revealing heterogeneously enhancing lesions in the liver suggestive of hepatic metastasis.
Fibreoptic bronchoscopy revealed deviation of trachea to left with narrowing of the right bronchial tree. There was no evidence of endobronchial mass in the visualised segments.
A 2D ECHO (Echocardiography) done revealed mild Mitral Regurgitation (MR), moderate Tricuscpid Regurgitation (TR), severe pulmonary hypertension, Pulmonary Artery Systolic Pressure (PASP-70 mmHg) with normal Left Ventricular (LV) systolic function.
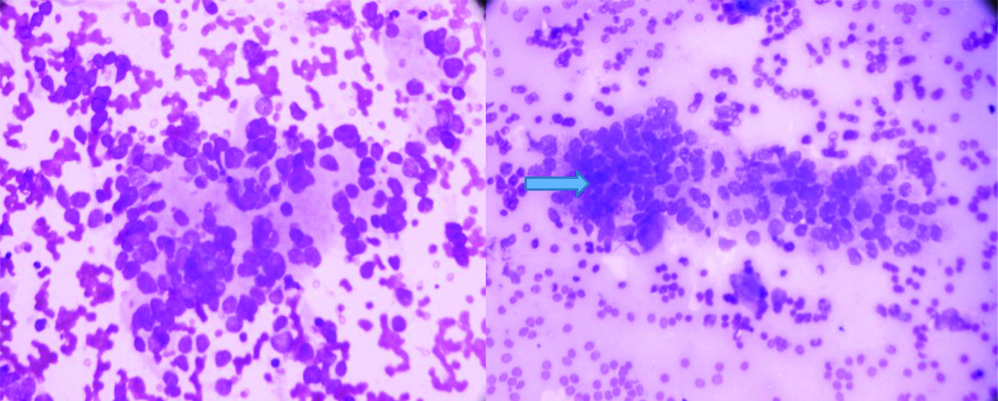
Ultrasonography (USG)-guided biopsy was done from the right-sided lung mass. Tissue specimen, subjected to Haematoxylin and Eosin (H&E) staining, showed predominantly small and occasionally intermediate-sized cells placed in cohesive cell sheet with peripheral decohesion and pseudoglandular structures, these cells carried hyperchromatic nuclei with high N:C ratio with mild pleomorphism, nuclear moulding and fine vesicular chromatin in few and peppersalt chromatin, suggestive of SCLC [Table/Fig-4]. Immunohistochemistry was advised but was not done due to financial constraints.
Hyperchromatic nuclei with increased N:C ratio and peppersalt chromatin, suggestive of Small Cell Carcinoma of Lung (SCLC) on H&E staining on 40x magnification.
Her Karnofsky Status was calculated as 90. Oncologist’s opinion was sought and palliative chemotherapy consisting of three cycles of Carboplatin (on day 1) and Etoposide (day 1-3) was advised, following which she would be reassessed. Patient is under follow-up after receiving two cycles of chemotherapy and her condition has not changed significantly with her weight, saturation in room air (94%) and chest x-ray similar to that found at the time of diagnosis.
Discussion
Whitening out of hemithorax is termed as Opaque Hemithorax which indicates the presence of a significant disease with accompanying loss of physiological functions. Differentials include Massive Pleural Fluid Effusion, Malignancies, Lung Aplasia, Agenesis of Lung and Lung Collapse [1]. Different causes that may present as an opaque hemithorax may be divided into malignant or benign. Of these, causes that lead to contralateral shift of the mediastinum include massive pleural effusion and malignancy, with the former being more common. Based on clinical findings such as presence of haemoptysis, hoarseness of voice, clubbing, loss of weight/appetite, a chronic aetiology like malignancy of the lung may be suspected. Lung cancer, a highly invasive, rapidly metastasising form of malignancy is the most common form of cancer diagnosed worldwide. Lung cancer accounts for more deaths than any other type of cancer [2]. Tobacco smoking is a major risk factor for development of malignancy of lung in Indian men. In Indian women, the association isn’t strong indicating the possibility of other risk factors such as air pollution (biomass exposure), hormone therapy. Lung cancer symptoms and signs are usually unspecific and at the time of presentation, the disease is at an advanced stage. Majority of the patients present with symptoms such as cough, haemoptysis, dyspnea, due to the central location which is characteristic of SCLC, affecting the central large bronchi [3].
SCLC in non-smokers is a very infrequent disease and accounts for 12-19% of total cases of lung cancer [4], and is usually diagnosed later in life than other lung cancer histological types. SCLCs rapidly grow and early metastasis to mediastinal lymph nodes has been found. Pressure on mediastinal structures may lead to superior vena cava obstruction, hoarseness and stridor. In spite of advances in recent years in terms of diagnosis, molecular changes, and therapeutic interventions, the outcomes of the lung cancer patients remain poor [5]. Treatment of choice for patients diagnosed with stage LA disease (T1N0) is a combination of radiotherapy and chemotherapy, although surgical resection may be considered. Management of tumors confined to the hemithorax, the mediastinum or supraclavicular lymph nodes involves combination of thoracic radiotherapy and platinum-based chemotherapy given with curative intent. Extensive-stage SCLC remains incurable with current treatment modalities, and patients are given palliative chemotherapy regimen involving a platinum-based regimen [6]. In contrast to commonly reported literature, the index case presented with predominant complaint of dry cough, and was relatively asymptomatic despite the extensive spread of the disease. Biopsy from the mass was confirmative of histological type of lung cancer and chemotherapy was given as per standard treatment protocols.
Conclusion(s)
Small cell lung cancer in non-smokers is infrequent and has an aggressive course. Survival is poor, even in patients diagnosed with a limited disease. Treatment modalities mainly include palliative chemotherapy in patients with extensive disease.
Author Declaration:
Financial or Other Competing Interests: None
Was informed consent obtained from the subjects involved in the study? Yes
For any images presented appropriate consent has been obtained from the subjects. Yes
Plagiarism Checking Methods: [Jain H et al.]
Plagiarism X-checker: Jul 09, 2020
Manual Googling: Aug 25, 2020
iThenticate Software: Sep 25, 2020 (4%)
[1]. Vaidya VN, Vohra PA, Ghugare BW, Opaque hemithorax: Clinical, histological and radiological assessment of 30 cases at a tertiary care hospital- A preliminary studyW Afr J of Radiology 2017 24(1):34-37.10.4103/1115-3474.198090 [Google Scholar] [CrossRef]
[2]. Ferlay J, Colombet M, Soerjomataram I, Mathers C, Parkin DM, Piñeros M, Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methodsInt J Cancer 2019 144(8):1941-53.10.1002/ijc.3193730350310 [Google Scholar] [CrossRef] [PubMed]
[3]. Torres-Durán M, Ruano-Ravina A, Kelsey KT, Parente-Lamelas I, Provencio M, Leiro-Fernández V, Small cell lung cancer in never-smokersEuropean Respiratory Journal 2016 47(3):947-53.10.1183/13993003.01524-201526699724 [Google Scholar] [CrossRef] [PubMed]
[4]. Govindan R, Page N, Morgensztern D, Read W, Tierney R, Vlahiotis A, Changing epidemiology of small-cell lung cancer in the United States over the last 30 years: Analysis of the surveillance, epidemiologic, and end results databaseJCO 2006 24(28):4539-44.10.1200/JCO.2005.04.485917008692 [Google Scholar] [CrossRef] [PubMed]
[5]. Noronha V, Pinninti R, Patil VM, Joshi A, Prabhash K, Lung cancer in the Indian subcontinentSouth Asian J Cancer 2016 5(3):95-103.10.4103/2278-330X.18757127606290 [Google Scholar] [CrossRef] [PubMed]
[6]. Simon GR, Turrisi A, American College of Chest Physicians. Management of small cell lung cancer: ACCP evidence-based clinical practice guidelines (2nd edition)Chest 2007 132(3 Suppl):324S-39.10.1378/chest.07-138517873178 [Google Scholar] [CrossRef] [PubMed]