Neurocysticercosis (NCC) is the most common parasitic disease affecting the human brain, and is also the most commonly identified cause of new-onset seizures in several regions of the world, including India [1]. Based on the results of a large community driven survey in Southern India, the prevalence of NCC as a cause of epilepsy in India, has been calculated to be one per 1000 population [2,3]. Thus, at least 1.2 million people in India are symptomatic with epilepsy due to NCC. The most common form of the disease in India is the SCG which is seen in up to sixty percent of patients [4].
Materials and Methods
This observational study with a case control design was carried out over a period of 4 years, from June 2015 to June 2019, at two Tertiary Care Hospitals in Mumbai and Bangalore. The study design was approved by the IEC at both the institutes (Asvini/IEC/2015/146 and IEC/CHAFB/2016/24). Only patients that gave written informed consent were enrolled in the study.
Sample size calculation was done using Fleiss Statistical method for Rates and Proportions (formulas 3.18 and 3.19) for an unmatched case control study [5]. The study was designed for a two-sided Confidence level of 95 (1- Alpha) and power of 80% to detect a significant difference. The hypothetical proportion of cases and controls having the exposure were taken as 30% and 10%, respectively. With the ratio of cases to controls at 1.25:1, a sample size of 124 was calculated (74 cases and 50 controls).
During this period a total of 246 patients underwent contrast MRI of the brain for evaluation of seizures, headache or raised Intra-Cranial Pressure (ICP) and focal neuro-deficits. The recruitment of patients into the study is shown in the Flow diagram. Seventy four (74) patients had MRI evidence of parenchymal/extra-parenchymal NCC lesions based on Del Bruto OH, criteria and were included in the study for further evaluation [Table/Fig-1] [6].
Flow diagram showing entry of patients in the study protocol.
Data of these patients was entered in a pre-designed detailed proforma with focus on seizure semiology, demographics, dietary habits, imaging patterns, serologic status and response to cysticidal therapy. Fifty unmatched controls with Idiopathic epilepsy, that were on OPD follow-up with normal neuroimaging, were also recruited in the study, so as to establish the sensitivity and specificity of the commonly used serologic test (IgG antibody) for NCC.
Apart from routine Haemogram and Biochemistry, all cases were subjected to Chest X-ray and Ultrasonography (USG) abdomen and rapid Human Immunodeficiency Virus (HIV) antibody test. A dilated fundus exam was done in all cases to look for ocular NCC. A 3 mL blood sample was also drawn to test serum for ELISA based IgG Anti-cysticercal antibodies, in both cases and controls. A standard Gadolinium enhanced contrast MRI Brain protocol (T1, T2, FLAIR, Diffusion, Gradient Echo and T1 Contrast) was followed in all cases, on a 1.5 Tesla Seimens MRI machine.
All patients with viable parenchymal NCC lesions were given standard cysticidal therapy with Albendazole (15 mg/kg per day for 2 weeks) under steroid cover, along with anti-epileptic drugs when indicated, as per clinician discretion. Disability at follow-up was assessed using the mRS [7].
Statistical Analysis
Data was entered and analysed using SPSS software ver. 21.0. Pearson’s Chi-square test was used to compare dependent variables. The p-values <0.05 were accepted as statistically significant.
Results
Clinical Profile and Demographics
The average outpatient and Inpatient load of these two study centers was 2500 to 3000 patients per year, of which 50 to 60 new cases of NCC are diagnosed per year. This gives the disease an annual incidence of 2%.
A total of 246 patients were screened with MR Imaging, and after detailed evaluation, 74 patients with established NCC were eligible to be enrolled in the study [Table/Fig-1]. Males constituted 81% (60) of the study population. Maximum cases 55% (41) were in the age group of 20-30 years. In terms of social strata, all patients belonged to a middle income or higher middle income group, being from armed forces community. In dietary preferences, 86% (64) patients were mixed diet consumers. Among presenting symptoms, 91% cases (68) presented with seizures. One patient had features of meningitis with multiple cranial nerve palsies, and another had para-paresis due to spinal NCC. Details of demography and clinical profile are given in [Table/Fig-2].
Demography and clinical features of cases with NCC (N=74).
| Parameter | N (%) |
|---|
| Demographic characterstics |
| Sex | |
| Male | 60 (81%) |
| Female | 14 (19%) |
| Age | |
| 20-30 yrs | 41 (55%) |
| 31-40 yrs | 20 (27%) |
| >50 yrs | 13 (18%) |
| Dietary habits | |
| Mixed diet | 64 (86%) |
| Vegetarian | 10 (14%) |
| Clinical characterstics |
| Presentation | |
| Seizures | 68 (91%) |
| Headache | 08 (11%) |
| Focal deficits | 04 (5%) |
| Seizure type | |
| GTCS | 57 (84%) |
| Focal seizures | 07 (10%) |
| CPS | 02 (3%) |
| Status epilepticus | 02 (3%) |
GTCS: Generalised tonic clonic seizures; CPS: Complex partial seizures
Neuroimaging
All patients underwent a standard Contrast MRI Brain as part of the study protocol and a wide spectrum of NCC lesions in different stages of evolution and healing were noted. In the entire study, 42% (31/74) had SCG, of which 23% (7/31) were purely calcified lesions. The imaging findings for SCG are summarised in [Table/Fig-3]. The pattern of Multiple NCC lesions on MRI Brain are depicted in [Table/Fig-4]. The wide spectrum of NCC lesions is brought out in [Table/Fig-5]. Two patients had extra-parenchymal NCC lesions in the intra-ventricular and subarachnoid space with complications of hydrocephalus and meningitis [Table/Fig-6]. One patient in the study had a spinal NCC lesion and presented with para-paresis [Table/Fig-7].
Pattern of Solitary NCC lesions on MRI in the study.
| Solitary cystisercal granuloma | Numbers (n) | Percent (%) |
|---|
| Calcified | 7 | 23 |
| Colloid | 6 | 19 |
| Nodular | 4 | 13 |
| Vesicular | 14 | 45 |
| Total | 31 | 100 |
Pattern of MRI with multiple NCC lesions.
| Multiple cystisercal granuloma | Numbers (n) | Percent (%) |
|---|
| Calcified + Colloid + Nodular | 7 | 16 |
| Colloid+ Vescicular | 6 | 14 |
| Nodular + Granular | 4 | 9 |
| Vesicular + Nodular | 11 | 26 |
| Calcified | 7 | 16 |
| Vesicular | 5 | 12 |
| Extra parenchymal/Spinal NCC | 3 | 7 |
| Total | 43 | 100 |
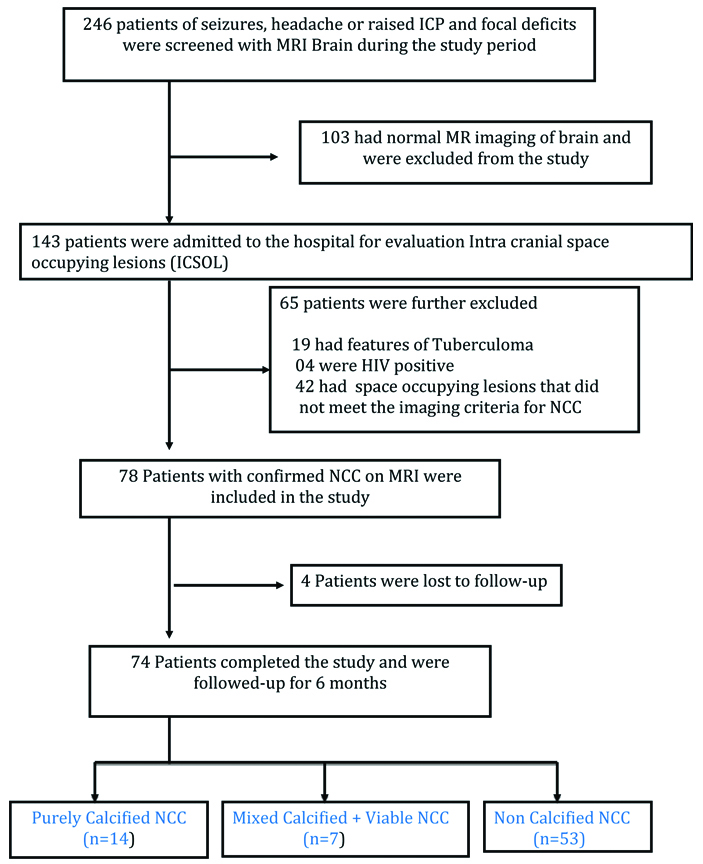
Different stages of NCC- Axial FLAIR (a) and T1 post contrast (b) showing NCC in the colloid vesicular stage (arrows) as a ring enhancing lesion with surrounding oedema. Axial T1 (c) and NCCT (d) showing extensive neurocysticercosis (NCC) in different stages. Scolex is visualised in some lesions (thin arrows). Calcification of lesions is noted on CT scan (Thick arrows).
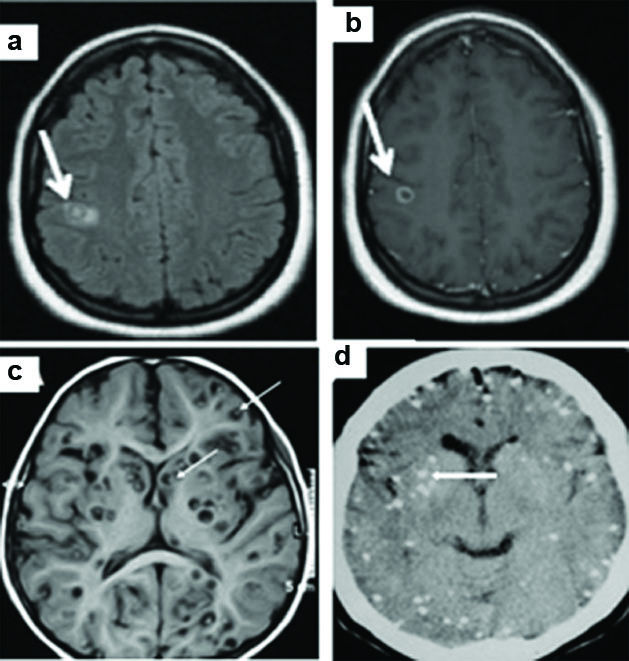
Intra-ventricular neurocysticercosis (NCC) with meningitis . MRI Brain (a) Axial 3D CISS (b) T2 TSE (c) Axial T1 & (d) Coronal T1 post contrast, showing cystic lesion within the 4th ventricle with eccentric nodule (thin arrows) and hydrocephalus. Diffuse irregular pachymeningeal thickening is noted in the post contrast images (thick arrows). Axial FLAIR (e) and T1WI (f) showing extensive cisternal cysts (arrows).
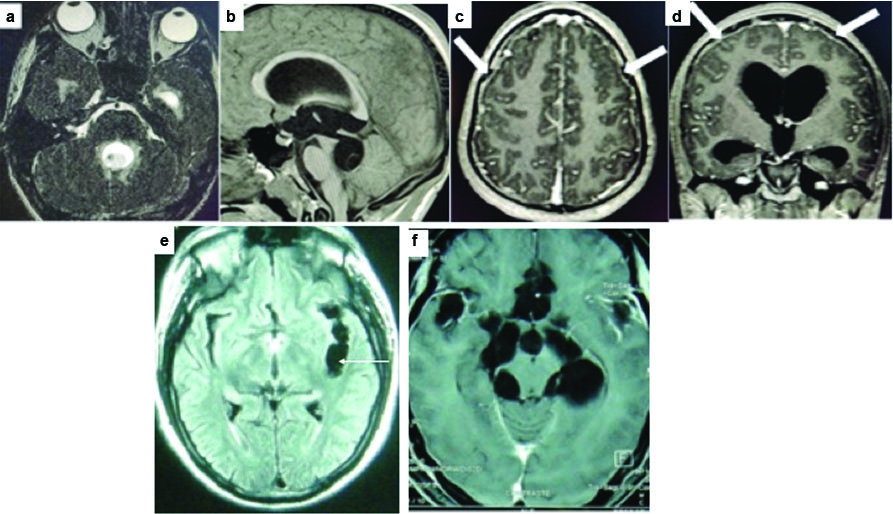
Intramedullary cysticercus in the cervical spine. Sagittal T2 (a) and T1 post contrast (b) and axial T2 and (c) T1 post contrast, showing smooth ring enhancing cystic lesion in the cervical cord (arrows) with surrounding cord oedema.
Serology
All 74 cases with NCC and 50 unmatched healthy controls were subjected to anti-cysticercal IgG antibody testing using standard ELISA kits. The results are summarised in [Table/Fig-8]. Only 26% (19/74) cases and 8% (4/50) controls were positive for the IgG antibody, giving the test a sensitivity of 26% (95% CI 15.7-5.6), and a specificity of 92% (95% CI 84.4-99.5). This translates into a PPV of 83% (95% CI 67-98), NPV of 45% (95% CI 35.8-55.2), giving a positive likelihood ratio of 3.21. (When compared with Contrast MRI Brain, which was used to confirm the diagnosis of NCC).
Cysticercal antibody testing in cases and controls.
| Ig Anti-cysticercus Ab | NCC (Cases) % | Healthy controls (%) |
|---|
| Positive | 19 (26%) | 4 (8%) |
| Negative | 55 (74%) | 46 (92%) |
| Total | 74 | 50 |
A likelihood ratio of 3.2 suggests that the yield of the antibody test is low, and there will only be a small shift between pre- and post-test probability, when this test is used in a clinical setting to diagnose NCC.
Authors compared the sero-positivity among calcified lesions on imaging 3/14 (21%), and non-calcified lesions 16/60 (27%). Despite higher sero-positivity in non-calcified lesions, it did not reach statistical significance (p=0.686) [Table/Fig-9].
Comparing sero-positivity between calcified and non-calcified lesions on MR Imaging.
| Ig G Cysticercus Ab test | Groups | Total | Chi-Sq | p-value |
|---|
| Calcified | Non calcified |
|---|
| Positive | 3 | 16 | 19 | 1.1632 | 0.686 |
| Negative | 11 | 44 | 55 |
| Total | 14 | 60 | 74 |
Further, comparing sero-positivity between patients with solitary lesions (SCG) on imaging (29%), with those having multiple lesions (23%), again there was no statistically significant difference (p=0.575) [Table/Fig-10].
Comparing sero-positivity between single and multiple NCC.
| Ig G Cysticercus Ab test | Groups | Total | Chi-Sq | p-value |
|---|
| Solitary | Multiple |
|---|
| Positive | 9 | 10 | 19 | 0.315 | 0.575 |
| Negative | 22 | 33 | 55 |
| Total | 31 | 43 | 74 |
Chi-Sq: Chi-Square Test
Treatment and Outcomes
All patients with only calcified lesions on MRI 19% (14/74), (7 solitary and 7 multiple), were not given cysticidal therapy, and managed symptomatically with Anti-epileptic drugs based on physician and institutional preferences. In patients with Extra-parenchymal NCC (2/74) and Spinal NCC (1/74), high dose steroids were used initially for 3-4 weeks, to control oedema and inflammation around the lesions. Steroids could be gradually tapered over 3-6 months in 2 cases. However, one case had recurrent meningitis and hydrocephalus [Table/Fig-7] on steroid tapering, requiring repeated VP shunts procedures, and transition to long term immune suppression with Azathioprine.
In the remaining patients (57/74), who had single or multiple viable parenchymal NCC lesions in various stages of evolution, Cysticidal therapy (Tab Albendazole 15 mg per kg/day for 14 days), under cover of steroids was given on an in-patient basis. This was combined with anti-epileptic drugs in appropriate doses based on physician preferences. This therapy was well tolerated in majority (54/57) of these cases, except. 2/57 developed recurrent seizures and Epilepsia Partialis Continua (EPC) and 1/57 developed severe headache and raised ICP, requiring early stoppage of Albendazole. They improved with steroids and anti-oedema measures and were not re-treated with cysticidal drugs.
At follow-up imaging after 6-9 months, 50/54 (92%) of the treated cases had a good clinical and radiologic response with resolved or calcified lesions, and good seizure control with single anti-epileptic drug. However, 4/54 had persisting or new lesions with increasing peri-lesional oedema, requiring a repeat course of combination cysticidal therapy (Tab Albenadazole 15 mg per kg/day for 21 days + Praziquantal 50 mg/Kg/day × 14 days) again given under steroid cover. These cases showed a good clinical and radiological response thereafter. Majority patients (95%) with parenchymal NCC (71/74) had good clinical outcomes (mRS-0-1), and 81% (66/74) had adequate seizure control with a single AED. Among the two patients with extra parenchymal NCC, one responded well to short term steroids and was mRS-2 at follow-up, however one with intra-ventricular NCC and hydrocephalus requiring VP shunt and immune-suppression with Azathioprine. He had significant residual ataxia and gaze impairment (mRS-3). The lone patient with spinal NCC, did respond to steroid therapy, and was unwilling for surgery. Patient had a residual grade 4/5 para-paresis with a mRS-3 at follow-up.
Discussion
NCC is prevalent in all parts of India and the density has been found to be the highest in the northern States of Bihar, Orissa, Uttar Pradesh and Punjab [8]. During this study conducted at two Tertiary Care Hospitals in Southern India, the incidence of 2% for new cases of NCC, was similar to data from other hospital based studies carried out at Dehradun and Vellore [9,10].
A significant majority of the cases, 55% population belonged to the young age group of 21-30 years. The infection tends to affect the younger and productive age groups more, leading to stigma of epilepsy and also loss of highly trained human resource. Previous studies have not found any dietary preferences significantly associated with the prevalence of the disease [2,4,10]. However, this study comprised of 86% mixed diet consumers and no consumers of pork or beef. This just seems to reflect the normal dietary preference of South Indian population and need not necessarily have a causal association with NCC. Previous studies from India have documented the prevalence of seizures amongst the patients of NCC to be around 75 to 90% [10,11]. In the present study, prevalence of seizures was similar at 91%, with the remaining patients presenting with headache, raised ICP and focal neurological deficits.
Parenchymal NCC was seen in 71/74 (96%) and Extra-parenchymal and spinal NCC was uncommon and seen in only 3/74 (4%). This is similar to trends observed in other studies from India and Mexico [10,12]. In terms of the type of parenchymal lesions seen on MRI, majority of lesions were the vesicular forms in 30% (22/74), followed by Calcified lesions in 28% (21/74) and the least common was Colloid forms in 17.5% (13/74). A similar trend has been observed in previous studies [13,14]. In the entire study, 42% (31/74) had SCG, of which 20% (7/31) were calcified lesions. This re-enforces the fact that the inactive looking and calcified lesion can still result in remote symptomatic seizures due peri-lesional gliosis and scarring that may not be easily visible on conventional MRI. It needs special MR sequences like Magnetisation Transfer (MT) and serial T2 Relaxometry (T2R), to bring out the disruption in neural architecture around the calcified lesions [15].
This study is unique in that, for the first time the authors used a case-control design to evaluate the utility of the commonly ordered Anti-cysticercal (IgG) antibody test in evaluation of NCC cases. It was observed that, only 26% (19/74) cases and 8% (4/50) controls were positive for the anti-cysticercal IgG antibody, giving the test a very low sensitivity of 26% and reasonable specificity of 92%. These values are lower than what was found in the earlier studies [16,17]. However, this test only detects the presence of antibodies to the larval antigen and does not necessarily indicate the presence of active disease. Hence, it may be positive in persons with exposure to the parasite antigen, those with past treated disease (antibodies may persist up to one year after successful treatment) and also those with disease outside the brain [18]. There will be a large number of asymptomatic persons in endemic regions who are seropositive with the ELISA but do not have the disease. In a large community based study from Vellore district, the sero-prevalence was around 15 per cent among asymptomatic persons [19]. The IgG antibody test is usually positive in patients with multiple non-calcified parenchymal lesions, intraventricular and subarachnoid NCC but has a low sensitivity in patients with SCG (around 60%) and is frequently negative in patients with solitary calcific parenchymal lesions [20]. Most of these studies are either community based sero-prevalence data, or done on MR imaging based diagnosis of NCC. The authors could not find a case control study on the subject.
Statistical analysis gave this antibody test a PPV of 83% and NPV of 45%, with a positive likelihood ratio of 3.21, for all forms of NCC in this study. On further analysis, the sero-positivity among non-calcified lesions was 27%, which was slightly higher than what was noted in calcified lesions (21%). However, this difference was not statistically significant (p=0.686) and suggest similar yield of the test in both calcified and viable lesions. These results suggest that the antibody test when negative does not rule out the disease with certainty (46% NPV), but a positive test helps in supporting a diagnosis of NCC with (83% PPV). However, with a low likelihood ratio of 3.2, this test on its own cannot be used to make the diagnosis of NCC, and MRI Brain still remains the main diagnostic tool. Previous data, confirm similar trends in other community based studies [21-23].
The response to therapy with cysticidal drugs in Parenchymal NCC was quite favorable in this study with 92% of treated cases showing a good clinical and radiologic response to a single 2 weeks course of Albendazole therapy. However, 8% required a second extended 3 weeks course of Albendazole therapy combined with Praziquantal therapy for 2 weeks. A similar trend of better response by combining the two cysticidal therapies, with a shorter duration of treatment, has been observed in various studies looking at treatment of NCC [24-26].
In this study, 4.5% patients had extra-parenchymal and Spinal NCC, which are challenging to treat. These cases showed steroid dependence for months, and one case with Inter-ventricular NCC had recurrent meningitis and hydrocephalus requiring VP shunts and transition to long term immune suppression with Azathioprine. Earlier studies have reported Extra-parenchymal NCC to constitute 5-10% of the total burden of NCC [27]. These can be either Intraventricular NCC, which may block the circulation of Cerebrospinal Fluid (CSF) causing obstructive hydrocephalus, or in the subarachnoid space where they tend to grow and expand their membranes, forming vesicular clusters (Racemose NCC) [28]. There are no randomised studies for treatment of Ventricular and Sub-arachnoid NCC and no standardised treatment regimens exist. Paradoxically, Albendazole and Praziquantel treatment provokes host inflammatory responses directed at the injured/dead parasites, resulting at times in disease deterioration [29]. Control of inflammation using short-term corticosteroids is generally well tolerated, but symptoms frequently recur after tapering of steroids, requiring transition to long term immune suppression with agents like Methotrexate, Azathioprine or Infliximab [30].
Patients with parenchymal NCC had a uniformly good clinical and radiological outcome in 95% cases, whereas remaining 5% cases with extra-parenchymal and spinal NCC had long term immunological complications, with increased moribity (mRS>2). The higher morbidity and disability in extra-parenchymal and spinal NCC is supported by literature from previous case studies [12,31,32].
Limitation(s)
Firstly, the present study was done at two Armed forces hospitals with a male predominant population, therefore the data may not be representative of the general population. Secondly, due to financial constrains, the commercially available and cheaper ELISA (IgG antibody) test was used to study the serology of NCC patients. However, the more accurate Enzyme-linked Immune-Electrotransfer Blot (EITB) test, may have given a better yield for the serological studies. Thirdly, contrary to convention, the ratio of cases to controls was kept at 1.25:1, resulting in higher number of cases, as compared to controls in this study.
Conclusion(s)
Neurocysticercosis (NCC) continues to be an important tropical infection and public health problem, which contributes significantly to the burden of epilepsy in our country. Modern contrast MR imaging has helped immensely in early diagnosis and treatment of this disease. The commercially available ELISA tests do not add significantly to the diagnosis and management of NCC. Majority of the patients tolerated cysticidal therapy well (under steroid cover), with favourable clinical and radiological outcomes. Extra-parenchymal and spinal forms of NCC remain a clinical challenge to diagnose and treat, with paucity of published literature and increased long term morbidity.