Introduction
The number of students in health science education is increasing day by day due to mushrooming of the private institutes. Hence, there is an increase in number of animals that have been used for animal experimentation irrespective of any known significance.
Aim
To assess the Knowledge, Attitude and Practice of health science students/researchers on status of usage of animals, 3R’s strategy (Reduction, Refinement, Replacement) and their alternatives for pharmacological studies.
Materials and Methods
This was a cross-sectional paper-based survey that was conducted between June 2019 to September 2019 using a semi-structured questionnaire to assess the knowledge, attitude and practice of the students. The study was conducted at the Guru Gobind Singh Medical College and Hospital, Faridkot and ISF College of Pharmacy, Moga, Punjab, India. The questionnaire consisted of three domains: Socio-demographic and professional characteristics, Knowledge (10 questions), attitude and practice of participants (9 questions). Out of 440 questionnaires distributed to the participants, 310 filled the survey (response rate was 70.5%) and were included in the study. The study participants comprised of MBBS interns, Junior residents/MD students, B pharmacy students, M pharmacy students and the PhD scholars.
Results
Majority of the students belonged to the age group of 19-23 years. It was observed that 79.6% (n=247) students didn’t know about the 3Rs (i.e., Reduction, Refinement, Replacement) Strategy. A 39.6% (n=123) respondents knew about the various alternatives to animal experimentation. Majority of the students 90.9% (n=282) believed that animal experiments are useful for medical research and human benefit.
Conclusion
There was an almost complete lack of knowledge among participants regarding alternative animal models and 3R’s strategy. Therefore, there is a need to incorporate education intervention about alternative animal use in their curriculum.
Introduction
The use of animals in experiments is known as animal testing. Sometimes, it is also termed as animal research, animal experimentation and in vivo testing [1,2]. Animal use is still prevalent for experimental purposes in pharmaceutical companies, medical schools, universities, commercial facilities in India that provide animal-testing service to research industry [3-6]. Animals are mainly used in research to answer some questions of great clinical importance, such as a new treatment for a disease, new indication of an old drug as well as in pharmaceutical companies whenever a new molecule comes into the picture [7-9]. Animal testing always remains on the forefront for establishment of safety and efficacy of the new drug molecules and are often carried out in preclinical trials [10,11]. The current scientific data reflects that the annual use of vertebrate animals exceeds a 100 million in number whereas more than 80 million rats, mice are being used in the United States for experimental purposes every year [12]. In India, about 1/3rd of the experiments are being conducted on small animals such as rats, fish, amphibians, mice, frogs and reptiles etc., [13]. Now-a-days, animal sacrifice is a debatable and serious matter of concern due to the fact that pain, distress, death of animals for animal experiments may lead to compromising experiment results [14]. In today’s world many health care professionals are debating either in the favour of animal experiments or against animal experiments (Institute for Laboratory Animal Research of the United States has argued that animal research cannot be replaced by computer models however, sophisticated as they fail to replicate the extremely complex interactions between molecules, cells, tissues, organs, organisms and the environment) [15]. On the contrary, Government is setting up various laws and regulation with the aim of regulating animal experiments to avoid unethical and unnecessary use of animals [16].
Although various laws and rules are being framed with respect to animal experiments yet the use of animals for research purpose is increasing [17]. The increase in animal research is due to the fact that the number of students in health science education is increasing day by day due to mushrooming of private institutes in India and the fact that they want to attract the students/clients for their business by showcasing research [18,19]. Due to this the number of pseudo researchers has disproportionally increased because of redundant research without any aim and novelty [20]. In addition, various regulatory bodies such as Drug Controller General of India (DCGI) and Indian Council for Medical Research (ICMR) have made the toxicity studies mandatory for pharmaceutical research which has led to increased use of animals for various experiments [21,22]. Toxicity studies are being carried out irrespective of knowing the significance or any potential background of the newly developed active pharmaceutical ingredient [23,24]. Hence, various animals like mice, rats, hamsters, rabbits, fish (such as zebra fish, trout), birds (such as chicken), guinea pigs, amphibians (including xenopus frogs), primates, dogs, cats, etc have been used in research since time immemorial [25].
Despite knowing the reality that animal models are not predictors of human response and some of the tests are outdated, the costs outweigh the benefits, animals have the intrinsic right, not to be used or harmed in experimentation and availability of alternatives to animal research every year millions of experimental animals are used all over the world [26,27]. Keeping in view the disproportionate use of animals, many organisations are working with the same objective to avoid the use of animals for research purposes [28,29]. The most important strategy known as 3R’s strategy was given by Russel and Burch to avoid the animal experiments in 1959 as shown in [Table/Fig-1] [6,30].
Representation of 3R’s strategy (Prepared by authors).
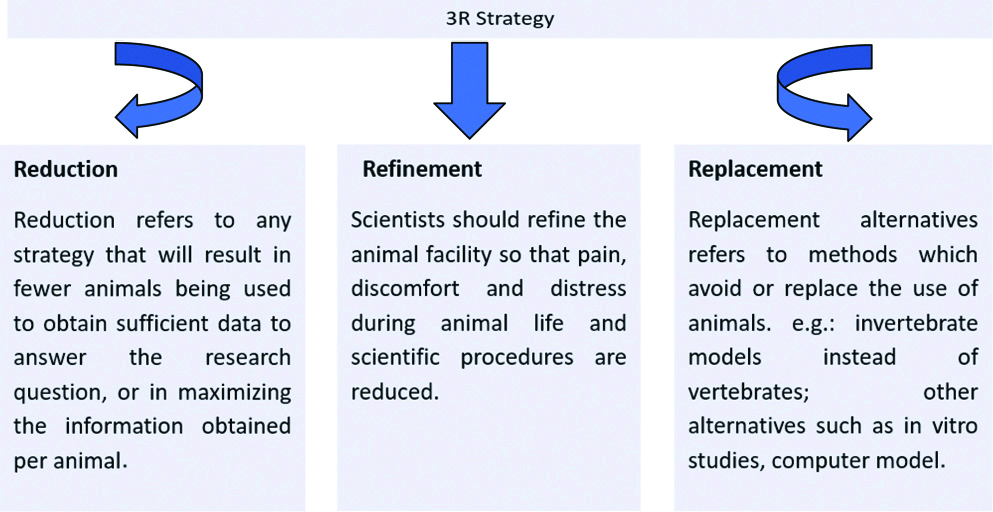
It has been hypothesised that animal research is very common among health science researchers but there is an almost a complete lack of knowledge regarding animal use and regulations related to animal experiments amongst researchers [31].
The present study was designed to assess the knowledge, attitude and practice of health science students/researchers on status of usage of animals, 3R’s strategy (Reduction, Refinement, Replacement) and their alternatives for pharmacological studies.
Materials and Methods
This was a cross-sectional paper-based survey that was conducted between June 2019 to September 2019 using a self-administered questionnaire. In this study, a paper-based questionnaire was developed through extensive literature review and the survey was distributed randomly to all the participants (MBBS interns, Junior residents, B Pharmacy students, M pharmacy students and the PhD scholars) to assess knowledge, attitude and practice of student’s on status of usage of animals, 3R’s strategy and their alternatives for pharmacological studies. The study was conducted at the Guru Gobind Singh Medical College and Hospital, Faridkot and ISF College of Pharmacy, Moga, Punjab, India.
The questionnaire consisted of three domains: Socio-demographic and professional characteristics, knowledge, attitude and practice of participants. First section assessed the socio-demographic details of the participants such as age, gender, qualification/academic degree. Second section consisted of 10 questions to evaluate the knowledge of participants regarding status of usage of animals, 3R’s strategy and their alternatives techniques and the last section consisted of nine questions that evaluated practices, attitude of participants towards animal’s experiments and different alternatives to animal experiments. Knowledge score was calculated with the help of responses, if the respondent answered the question correctly one mark was given for each correct answer. If the respondent answered the question incorrectly or didn’t answer the question then 0 score was given. Finally, the total score for each respondent was calculated.
This study targeted the students currently studying in the state of Punjab, India. The estimation of sample size was done with the help of Epi info software, there are approximately 700 medical interns, 10,500 B pharmacy students, 4250 M pharmacy students, 250 PhD research scholars and 300 postgraduate residents studying in different pharmacy and medical colleges of Punjab, India. The researcher took into account a 30% non-response rate and to achieved a confidence level of 95% and a 5% margin of error, the study sample size came out to be 312. The participants were randomly selected to participate in the study. Out of 440 questionnaires distributed only 310 participants (response rate was 70.5%) had filled the survey and returned and were included in the study. All participants were asked to fill informed consent prior to registration. The informed consent page presented two options (I agree/I disagree). Subjects who chose I agree option were included in the study and were allowed to fill the questionnaire, and subjects could withdraw their name at any time during the process. Participant had to fill out the questionnaire in the presence of investigator and was not allowed to refer to any information resources during answering the questions.
A semi-structured questionnaire was developed based on previous similar studies but keeping the Indian scenario in mind [32,33]. The developed questionnaire was validated using face and content validation methods by senior consultants, researchers working in pharmacology, pharmaceutics, microbiology, and medicine and research department to ensure its readability. Reliability was assessed using Cronbach’s alpha test and the value of alpha came out to be 0.6. Secondly, it was assessed for reliability, clarity and completion time through a pilot study that was pretested among 20 purposively selected respondents who were eventually excluded from the data analysis. Lastly, the survey questionnaire was distributed among the participants after revising it based on the obtained comments/feedback from the participants. The questionnaire focused on the form of use, the dignity of animals, the killing of animals, the health of animals, animal experimentation, improvements in animal genotypes, animal ecosystem and the social attitudes towards animals.
Results
Demographic Details of the Study Participants
A total of 440 questionnaires were distributed to the participants and among those 310 filled the questionnaires which was analysed for further results (response rate was 70.5%). As per the demographic profile, it was observed that 54.2% (n=168) of the students belonged to the age group of 19-23 years. 53% females (n=164) participated in the study. On the other hand, 47% (n=146) of the males participated in the study. Majority of the students were studying in undergraduate courses i.e., 31.6% (n=98) in MBBS (medical interns) and 36.1% (n=112) in B Pharmacy, students pursuing masters i.e., 6.5% (n=20) in M Pharmacy degree, and 24.5% (n=76) were pursuing MD, 4 (1.3%) were PhD scholars participated in the study. Socio-demographic details of the patient are presented in [Table/Fig-2].
Demographic details of population covered (n=310).
| Characteristics | Frequency (%) |
|---|
| Age (Years) |
| 14-18 | 8 (2.6%) |
| 19-23 | 168 (54.2%) |
| 24-28 | 110 (35.5%) |
| ≥29 | 24 (7.7%) |
| Gender |
| Male | 146 (47%) |
| Female | 164 (53%) |
| Academic degree |
| MBBS (Medical interns) | 98 (31.6%) |
| MD (Junior Resident) | 76 (24.5%) |
| B Pharmacy | 112 (36.1%) |
| M Pharmacy | 20 (6.5%) |
| PhD | 4 (1.3%) |
Various questions framed to assess the knowledge of the participants and their responses are shown in [Table/Fig-3]. Out of 310 respondent’s majority of respondents 79.6% (n=247) did not know about the full form of 3Rs, only 12.5% (n=39) answered the question correctly whereas 7.7% (n=24) answered incorrectly. A 56.4% (n=175) half proportion of the respondents (88%, n=273) did not know about the lower vertebrates such as Zebra fish that can be used as an alternative to experiments on higher vertebrates for most of the research experiments. 39.6% (n=123) respondents know about the alternatives to animal experiments.. Moreover, 65.4% (n=203) of the respondents knew about the reason behind banning cosmetic experiments on animals. On the other hand, 51.2% (n=159) students knew that animals have been euthanised during and after experimental procedure to avoid pain, sufferings, distress or lasting harm in animals. A 59.6% (n=185) knew about the prevention of cruelty to animals act. A total of 49.6% (n=154) of the students did not know about the different computer models that are used as an alternative to animal experiments.
Knowledge of students on status of usage of animals and their alternatives for pharmacological studies (n=310).
| Knowledge questions | Frequency |
|---|
| Correct responses | Incorrect responses | Don’t know |
|---|
| 1. Full Form of 3Rs? | 39 (12.5%) | 24 (7.7%) | 247 (79.6%) |
| 2. Which Lower vertebrate can be used as an alternative to higher vertebrates? | 31 (10%) | 6 (1.9%) | 273 (88%) |
| 3. Alternatives to animal experiments are being used in which science and research field? | 123 (39.6%) | 12 (3.8%) | 175 (56.4%) |
| 4. Why Humans are not be used for research as compared to animals? | 242 (78%) | 28 (9%) | 40 (12.9%) |
| 5. Which animals are used for experimentation? | 234 (75.4%) | 13 (4.2%) | 63 (20.3%) |
| 6. Animal protection act build up by which society? | 168 (54.1%) | 66 (21.2%) | 76 (24.5%) |
| 7. Why Cosmetics Animal Experiments are banned? | 203 (65.4%) | 32 (10.3%) | 75 (24.1%) |
| 8. Why animals have been euthanised during and after experimental procedure? | 159 (51.2%) | 28 (9%) | 123 (39.6%) |
| 9. Prevention of cruelty to Animal act enacted in which year and why? | 185 (59.6%) | 39 (12.5%) | 86 (27.7%) |
| 10. Which computer models can be used as alternative to animal experiments? | 102 (32.9%) | 54 (17.4%) | 154 (49.6%) |
Represents distribution of respondents according to their knowledge score [Table/Fig-4].
Distribution of respondents according to their knowledge score.
| Knowledge score | Frequency | Percent |
|---|
| 2 | 12 | 3.9 |
| 3 | 28 | 9.0 |
| 4 | 60 | 19.4 |
| 5 | 104 | 33.5 |
| 6 | 44 | 14.2 |
| 7 | 36 | 11.6 |
| 8 | 18 | 5.8 |
| 9 | 8 | 2.6 |
| Total | 310 | 100.0 |
The attitude and practice of students on status of usage of animals and their alternatives for pharmacological studies are shown in [Table/Fig-5]. Out of 310 respondents, 33.5% (n=104) respondents were studying about animal experiments alternatives in their academic degree and 79.4% (n=246) students were doing animal experiments in their lab.
Attitude and practice of students on status of usage of animals and their alternatives for pharmacological studies (n=310).
| Attitude and practice of students/researcher | Frequency |
|---|
| Agree | Disagree |
|---|
| 1. Are you studying animal experiments alternatives in lab | 104 (33.5%) | 206 (66.4%) |
| 2. Are you doing animal experiments in lab | 246 (79.4%) | 64 (20.6%) |
| 3. Do you think we can use animals for experiments as long as it is intended for a good cause rather than harm. If it is used for a good purpose it could be beneficial to both humans and animals. | 282 (90.9%) | 28 (9%) |
| 4. Animals in research must be treated at the same levels for their rights as human | 12 (3.8%) | 298 (96.1%) |
| 5. What do you think we use animals because they are not feeling pain and act as living machines and good sources of knowledge | 20 (6.4%) | 290 (93%) |
| 6. Live human beings are not sacrificed for medical research because they have more advanced mental abilities like they are intelligent than other animals | 247 (79.6%) | 63 (20.4%) |
| 7. If a human is not having mental abilities in some special cases such as babied or infants or any human suffering from brain disorders such as Alzheimer etc., can be used for research | 52 (16.7%) | 258 (83.3%) |
| 8. Is there any requirement of developing alternative research models that do not use animals or animal experimentation | 269 (86.7%) | 41 (13.2%) |
| 9. Will you go for different alternatives in animal experimentation | 267 (86.1%) | 43 (13.8%) |
Majority of the students 90.9% (n=282) believed that animal experiments can be used for research experiments as long as it is intended for a good cause rather than harm. If it is used for a good purpose it could be beneficial to both humans and animals. About more than three quarter of the participants 79.6 % (n=247) believed that human beings are not sacrificed for medical research because they have more advanced mental abilities like they are intelligent than other animals and 16.7% (n=52) they also believe and recommend that humans suffering from brain disorders such as Alzheimer’s etc., can be used for experiments. However, 86.7% (n=269) of the participants strongly agreed that there is requirement to develop alternative research models that do not use animals or animal experimentation. Most of the students i.e., 86.1% (n=267) of the population agreed to go for alternatives in animal experimentation.
It was also observed that concerning to academic degrees whether it was undergraduate courses or a master’s degree almost 80% of the students were performing animal experiments. But most of the undergraduate students were not aware about the alternatives of the animal experiments or bioethics.
Discussion
In present study, it was observed that about 90% of the participants believed that medical research involving animal experiments is very important for development of new drugs and is an integral part of medical research whereas the same proportion of the respondents (86.7%) felt that there should be alternative to use of animals for medical research. However, various studies suggests poor clinical and toxicological studies on animal models failed to predict human toxicological outcomes such as carcinogenicity and the results from animal models were frequently equivocal [34-39]. So, animal data may not generally be considered useful for these purposes. In continuation to it, 56.4% (n=175) of the study population were not aware of the methods to avoid the use of animals in medical research. They had poor knowledge for any existing strategies such as 3R’s strategy presented by Animal Welfare Society. Keeping the above mentioned points in mind, the animal experiments should be performed with respect to guidelines recommended for animal use by competent authorities.
A study conducted by Phillips CJC et al., recommended that European countries students are found to be more concerned for animal welfare than Asian countries students as there is a sociopolitical situation arising in regions rather than other differences [40].
In present study, it was also observed that while undergraduate students were doing experiments on animals involving frogs, rats, mice etc., most of them knew very little about the bioethics and animal or human biomedical research. Animal ethics is an issue as important as the human welfare. Most of the participants voted that animal ethical committee’s rules should be stringent enough as human ethical committee rules. The viewpoint of present study can be compared with study conducted by Knight A [34]. The competent authorities and organisation have recommended various alternatives for animal use that need to implement in an effective manner. Various tools are bioinformatics, computer models, in vitro cell cultures, enzymatic screens and model organisms [41-43]. Use of modern analytical techniques, data acquisition and statistical procedures to analyse the results of alternative protocols can provide dependable outcomes, yet more efforts are need to be undertaken for effective implementation of 3Rs strategy in India [44].
Conducting experiments on animals for medical purpose is a common practice in India. In the current situation in India, every institution has established institutional ethics committee for conducting experiments on animals. Students have to submit protocol of their research and have to take approval from their respective institution ethics committee prior to experiments on animals. Students have to calculate sample size of animals needed in their study and most of the times the ethics committee chairperson asked the students to decrease their sample size of animals for experiments [31]. Due to stringent rules and regulations for unnecessary experiments on animals, many students agreed to reduce sample size or go for abolishing animal use during experiments. This would be a disaster for medical research as animal serve as a building block for drug and medical research in humans [45,46].
Limitation(s)
The study was limited to just 310 medical and pharmacy students of Punjab and this served as a limitation of the study. Larger studies in diverse pharmacy and medical students across various India are needed to draw a greater perspective.
Conclusion(s)
Overall, we concluded that undergraduate pharmacy and medical students had very little knowledge about 3R’s strategy and other various alternatives for animal use. The use of integrated approaches is the need of the hour to reduce or avoid the involvement of animals in scientific procedures. There is a need of education intervention of 3R’s strategy and other available alternative models for animal use in their core curriculum.
[1]. Monamy V, Animal experimentation: A guide to the issues 2017 Cambridge University Press10.1017/9781316678329 [Google Scholar] [CrossRef]
[2]. Festing S, Wilkinson R, The ethics of animal researchEMBO Reports 2007 8(6):526-30.10.1038/sj.embor.740099317545991 [Google Scholar] [CrossRef] [PubMed]
[3]. Giridharan NV, Kumar V, Muthuswamy V, Use of animals in scientific researchIndian Counc Med Res 2000 :01-27. [Google Scholar]
[4]. Barnes CD, Eltherington LG, Drug dosage in laboratory animals: A handbook 1966 Univ of California Press [Google Scholar]
[5]. Tilp C, Kapur V, Loging W, Erb KJ, Prerequisites for the pharmaceutical industry to develop and commercialise helminths and helminth-derived product therapyInt J Parasitol-Par 2013 43(3-4):319-25.10.1016/j.ijpara.2012.12.00323291462 [Google Scholar] [CrossRef] [PubMed]
[6]. Törnqvist E, Annas A, Granath B, Jalkesten E, Cotgreave I, Öberg M, Strategic focus on 3R principles reveals major reductions in the use of animals in pharmaceutical toxicity testingPloS one 2014 9(7):e10163810.1371/journal.pone.010163825054864 [Google Scholar] [CrossRef] [PubMed]
[7]. Alini M, Eisenstein SM, Ito K, Little C, Kettler AA, Masuda K, Are animal models useful for studying human disc disorders/degeneration?Eur Spine J 2008 17(1):02-19.10.1007/s00586-007-0414-y17632738 [Google Scholar] [CrossRef] [PubMed]
[8]. EFSA Panel on Additives and Products or Substances used in Animal Feed (FEEDAP)Technical guidance: Tolerance and efficacy studies in target animalsEFSA Journal 2011 9(5):217510.2903/j.efsa.2011.2175 [Google Scholar] [CrossRef]
[9]. Williams LD, Burdock GA, Edwards JA, Beck M, Bausch J, Safety studies conducted on high-purity trans-resveratrol in experimental animalsFood Chem Toxicol 2009 47(9):2170-82.10.1016/j.fct.2009.06.00219505523 [Google Scholar] [CrossRef] [PubMed]
[10]. Ruggeri BA, Camp F, Miknyoczki S, Animal models of disease: Pre-clinical animal models of cancer and their applications and utility in drug discoveryBiochem Pharmacol 2014 87(1):150-61.10.1016/j.bcp.2013.06.02023817077 [Google Scholar] [CrossRef] [PubMed]
[11]. Mak IW, Evaniew N, Ghert M, Lost in translation: Animal models and clinical trials in cancer treatmentAm J Transl Res 2014 6(2):114 [Google Scholar]
[12]. Taylor K, Gordon N, Langley G, Higgins W, Estimates for worldwide laboratory animal use in 2005ATLA: Altern Lab Anim 2008 36(3):327-42.10.1177/02611929080360031018662096 [Google Scholar] [CrossRef] [PubMed]
[13]. Pereira S, Veeraraghavan P, Ghosh S, Gandhi M, Animal experimentation and ethics in India: The CPCSEA makes a difference 2004 32(1_suppl)ATLA: Altern Lab Anim:411-15.10.1177/026119290403201s6723581110 [Google Scholar] [CrossRef] [PubMed]
[14]. Carstens E, Moberg GP, Recognizing pain and distress in laboratory animalsIlar Journal 2000 41(2):62-71.10.1093/ilar.41.2.6211304586 [Google Scholar] [CrossRef] [PubMed]
[15]. Committee to Update Science M, National Research CouncilScience, Medicine, and Animals 2004 National Academies Press (US) [Google Scholar]
[16]. de Boo J, Hendriksen C, Reduction strategies in animal research: A review of scientific approaches at the intra-experimental, supra-experimental and extra-experimental levelsATLA: Altern Lab Anim 2005 33(4):369-77.10.1177/02611929050330040416185105 [Google Scholar] [CrossRef] [PubMed]
[17]. Olfert ED, Cross BM, McWilliam AA, Guide to the care and use of experimental animals 1993 OttawaCCAC [Google Scholar]
[18]. Cheney GR, Ruzzi BB, Muralidharan K, A profile of the Indian education system, prepared for the New Commission on the Skills of the American Workforce 2005 Nov Washington DC, United States [Google Scholar]
[19]. Baum M, Kabst R, How to attract applicants in the Atlantic versus the Asia-Pacific region? A cross-national analysis on China, India, Germany, and HungaryJ World Bus 2013 48(2):175-85.10.1016/j.jwb.2012.07.002 [Google Scholar] [CrossRef]
[20]. Altbach PG, Levy DC, Private higher education: A global revolution 2005 Sense publishers10.1163/9789087901035 [Google Scholar] [CrossRef]
[21]. Laurence DR, Bacharach AL, Evaluation of drug activities: Pharmacometrics 2013 Elsevier [Google Scholar]
[22]. Olson H, Betton G, Robinson D, Thomas K, Monro A, Kolaja G, Concordance of the toxicity of pharmaceuticals in humans and in animalsRegul Toxicol Pharmacol 2000 32(1):56-67.10.1006/rtph.2000.139911029269 [Google Scholar] [CrossRef] [PubMed]
[23]. Lumley CE, Walker SR, A critical appraisal of the duration of chronic animal toxicity studiesRegul Toxicol Pharmacol 1986 6(1):66-72.10.1016/0273-2300(86)90040-1 [Google Scholar] [CrossRef]
[24]. Saganuwan SA, Toxicity studies of drugs and chemicals in animals: An overviewBulg J Vet Med 2017 20(4):291-318.10.15547/bjvm.983 [Google Scholar] [CrossRef]
[25]. Baumans V, Use of animals in experimental research: An ethical dilemma?Gene Therapy 2004 11(1):S64-66.10.1038/sj.gt.330237115454959 [Google Scholar] [CrossRef] [PubMed]
[26]. Shanks N, Greek R, Greek J, Are animal models predictive for humans?PEHM 2009 4(1):210.1186/1747-5341-4-219146696 [Google Scholar] [CrossRef] [PubMed]
[27]. Bishop LJ, Nolen AL, Animals in research and education: Ethical issuesKennedy Inst Ethics J 2001 11(1):91-112.10.1353/ken.2001.000612166448 [Google Scholar] [CrossRef] [PubMed]
[28]. Croce P, (1999) Vivisection or science: An investigation into testing drugs and safeguarding health2nd edNew YorkZed [Google Scholar]
[29]. Bert B, Dörendahl A, Leich N, Vietze J, Steinfath M, Chmielewska J, Rethinking 3R strategies: Digging deeper into Animal Test Info promotes transparency in in vivo biomedical researchPLoS Biology 2017 15(12):e200321710.1371/journal.pbio.200321729240762 [Google Scholar] [CrossRef] [PubMed]
[30]. Richmond J, Refinement, reduction, and replacement of animal use for regulatory testing: Future improvements and implementation within the regulatory frameworkILAR Journal 2002 43(Suppl_1):S63-68.10.1093/ilar.43.Suppl_1.S6312388854 [Google Scholar] [CrossRef] [PubMed]
[31]. Pifer R, Shimizu K, Pifer L, Public attitudes toward animal research: Some international comparisonsSociety & Animals 1994 2(2):95-113.10.1163/156853094X0012611654358 [Google Scholar] [CrossRef] [PubMed]
[32]. Millett K, Lock R, GCSE students’ attitudes towards animal use: Some implications for biology/science teachersJ Biol Educ 1992 26(3):204-08.10.1080/00219266.1992.9655274 [Google Scholar] [CrossRef]
[33]. Pious S, Attitudes toward the use of animals in psychological research and educationPsychol Sci 1966 7:352-58.10.1111/j.1467-9280.1996.tb00388.x [Google Scholar] [CrossRef]
[34]. Knight A, Systematic reviews of animal experiments demonstrate poor contributions toward human healthcareRev Recent Clin Trials 2008 3(2):89-96.10.2174/15748870878422384418474018 [Google Scholar] [CrossRef] [PubMed]
[35]. Huff J, Chemicals studied and evaluated in long-term carcinogenesis bioassays by both the Ramazzini Foundation and the National Toxicology ProgramAnn NY Acad Sci 2002 982:208-30.10.1111/j.1749-6632.2002.tb04935.x12562639 [Google Scholar] [CrossRef] [PubMed]
[36]. Ennever FK, Lave LB, Implications of the lack of accuracy of the lifetime rodent bioassay for predicting human carcinogenicityRegul Toxicol Pharmacol 2003 38:52-57.10.1016/S0273-2300(03)00068-0 [Google Scholar] [CrossRef]
[37]. Knight A, Bailey J, Balcombe J, Animal carcinogenicity studies: 1. Poor human predictivityATLA: Altern Lab Anim 2006 34(1):19-27.10.1177/02611929060340011716522147 [Google Scholar] [CrossRef] [PubMed]
[38]. Bailey J, Knight A, Balcombe J, The future of teratology research is in vitroBiog Amines 2005 19(2):97-145.10.1163/1569391053722755 [Google Scholar] [CrossRef]
[39]. Olson H, Betton G, Stritar J, Robinson D, The predictivity of the toxicity of pharmaceuticals in humans from animal data- An interim assessmentToxicol Lett 1998 102(3):535-38.10.1016/S0378-4274(98)00261-6 [Google Scholar] [CrossRef]
[40]. Phillips CJC, Izmirli S, Aldavood SJ, Alonso M, Choe BI, Hanlon A, Students’ attitudes to animal welfare and rights in Europe and AsiaAnimal Welfare 2012 21(1):87-100.10.7120/096272812799129466 [Google Scholar] [CrossRef]
[41]. Ferrer-Costa C, Orozco M, de la Cruz X, Use of bioinformatics tools for the annotation of disease-associated mutations in animal modelsProteins: Structure, Function, and Bioinformatics 2005 61(4):878-87.10.1002/prot.2066416208716 [Google Scholar] [CrossRef] [PubMed]
[42]. Girard M, Interactive design of 3-D computer-animated legged animal motionIn Proceedings of the 1986 workshop on Interactive 3D graphics 1987 :131-150.10.1145/319120.31913116665644 [Google Scholar] [CrossRef] [PubMed]
[43]. Ekwall B, Silano V, Paganuzzi-Stammati A, Zucco F, Toxicity tests with mammalian cell culturesShort-Term Toxicity Tests for Non-Genotoxic Effects 1990 41:75-82. [Google Scholar]
[44]. Bayne K, Ramachandra GS, Rivera EA, Wang J, The evolution of animal welfare and the 3Rs in Brazil, China, and IndiaJournal of the American Association for Laboratory Animal Science 2015 54(2):181-91. [Google Scholar]
[45]. Arluke A, Hafferty F, From apprehension to fascination with ‘Dog Lab’: The use of absolutions by medical studentsJ Contempor Ethnogr 1996 25(2):201-25.10.1177/089124196025002002 [Google Scholar] [CrossRef]
[46]. Nerlekar S, Karia S, Harshe D, Warkari R, Desousa A, Attitude and knowledge of undergraduate medical students towards the use of animals in medical research: An exploratory studyJ Clin Diagn Res 2018 12(7):JC04-06.10.7860/JCDR/2018/32260.11768 [Google Scholar] [CrossRef]