Administration of intravenous fluid is one of the most common interventions in medicine [1]. Fluid resuscitation for hypovolemic shock is an integral part of acute medical management in the Intensive Care Unit (ICU) and Operation Theatre (OT) and in the emergency department. Colloids and crystalloids are also used as co-loading fluids to limit complications like hypotension following sympathetic blockade in the central neuraxial blockade [2,3]. RL, a balanced salt solution is the most common crystalloid fluid used for fluid resuscitation. It is also preferred as the fluid of choice for co-loading in patients following SAB. Commercially available colloids are widely recommended in several resuscitation guidelines and intensive care algorithms [4]. HES, a colloid have the potential to be an alternative to RL as a fluid for co-loading in patients undergoing SAB. This is so as smaller volumes are used as a co-loading fluid and that too for a smaller duration following SAB. HES preparations have lost its popularity due to reported complications like acute kidney injury and coagulopathy in critically ill patients. But, it is still recommended by the European Medicines Agency for ongoing use to treat haemorrhage in surgical patients and trauma cases [5]. Amylopectin, a highly branched compound of starch, resembles glycogen in structure is rapidly hydrolysed with a half (½) life of about 20 minutes. To make the amylopectin molecule more stable, hydroxyethyl glucose residues are substituted with hydroxyethyl groups mainly at positions C2 and C6. HES are identified by three numbers, e.g., 10% HES 200/0.5 or 6% HES 130/0.4. The first number indicates the concentration of the solution, the second represents the mean Molecular Weight (MW) expressed in kilo Dalton (kDa), and the third and most significant one is Molar Substitution (MS) [1].
But HES being a derivative of amylopectin (starch) has the potential to cause an increase in BSL. This is due to the breakdown of amylopectin by plasma alpha-amylase to glucose. As lactate in RL solution gets converted into glucose via the Cori cycle, RL also has some theoretical risk of increasing BSL. The stress response to surgery due to elevated levels of released catecholamines have the hyperglycaemic effect, so any added factor-like intravenous fluid used for co-loading may prove detrimental to the patient [2,6]. Studies on HES as co-loading fluid were mostly on its haemodynamic effect [7]. Not many studies have been done to find out the effect of HES on BSL during surgery [8,9] and the comparison with RL, the common intravenous fluid used for co-loading in this regard. It is always warranted to have a colloid in the armament of an anaesthesiologist when larger volume administration is an issue, even with a balanced intravenous fluid-like RL. But before advocating some drugs we must realise the effects of that in various conditions. HES is still available in the market for the specific use as mentioned earlier. So, it is always better to decide on options in critical situations where even a minor reduction in the volume of co-loading fluid will benefit the patient. We believe HES has that potential. Most of the studies used a lesser volume of HES to that of RL when comparing their effect keeping in mind the volume expansion effect of colloids [3]. The present study kept the same volume in all the groups to check the safety margin in terms of hyperglycaemia along with the proven effect like haemodynamic changes.
This prospective comparative study aimed to compare the effect on blood sugar in patients for surgeries under spinal anaesthesia in two different preparations of HES and RL. In this study, the primary objective was to compare the BSLs in all the three groups at various intervals after administration as a co-loading fluid with a secondary objective was to compare the haemodynamic changes in all the three groups.
Materials and Methods
This comparative observational study was carried out between August 2015 to July 2017 in a tertiary care teaching hospital, after taking approval from the Institutional Ethical Committee (27/IEC/2015) and written informed consent from the patients. A total of 90 patients aged between 18 to 64 years, of either sex undergoing lower abdominal and lower limb surgeries under the SAB and in American Society of Anaesthesiology (ASA) physical status I and II, were enrolled in this study. Patients with a history of known allergy or hypersensitivity against starch or RL, known cases of diabetes mellitus, chronic kidney diseases, history of coagulopathy, emergency surgeries, and patients on regular use of steroid and ascorbic acid were excluded.
During the study period using the consecutive sampling technique, out of 90 patients, initial 30 patients fulfilling study protocol were allocated into HES 200 group (Group A), the next 30 patients were given HES 130 group (group B) and the last 30 patients were allocated into the RL group (group C). All patients were co-loaded with either: 6% HES- 200 (Group A) 20 mL/kg, 6% HES-130 (Group B) 20 mL/kg, and RL (Group RL) 20 mL/kg respectively over 30 minutes, immediately after the SAB. Fluids administered for co-loading were at the prudence of the Anaesthesiologist conducting the case. Plasmalyte was used as maintenance fluid until the end of the surgery once co-loading was over. All patients were administered 2.2 mL to 2.6 mL of 0.5% Bupivacaine (heavy) depending on the age, height, and other concerning factors of the patient. No adjuvant was used in any of the cases. ASA Standard monitoring (Heart rate, Pulse, Non-invasive blood pressure, Temperature, and ECG) was established in all cases. Throughout the surgery capillary blood- glucose levels were monitored with Accu-Chek Performa glucometer (Roche Diagnostics India) at regular intervals. This was done by an Anaesthesiologist who was not aware of the group allocation. The baseline blood sample was taken just before co-loading and subsequently at 15, 30, 45, 60, 120, 180, and 240 min from the baseline reading.
Statistical Analysis
The sample size was calculated using the ‘G*Power3 free’ software and the findings of the previous study [10]. The effect size was assumed according to the Cohen’s guidelines for the social sciences using α=0.05 with a power of 80%, the total sample size was calculated as 81 for the statistical analysis of seven consecutive time points between different factors with a correlation of 0.5. After assuming a 10% drop out rate, 30 patients were allocated to each group. Data were analysed with Statistical Package for Social Sciences (SPSS) version 17.0. A Chi-square test was used to test the association between types of surgeries with three treatment groups. Analysis of Variances (ANOVA) was employed to compare the mean of BSL, Systolic Blood Pressure (SBP), Diastolic Blood Pressure (DBP) Pulse rate in three groups, Tukey’s test was used for post-hoc analysis and it was applied for pairwise comparison, p-value <0.05 was considered to be significant and p-value <0.001 was considered to be highly significant.
Results
All 90 patients completed the study except for one (01) patient in RL group who was excluded due to failed SAB and the requirement of conversion to general anaesthesia. A comparison of the mean age±standard deviation (in years) was done using the ANOVA test. The p-value calculated was 0.587. The mean weight in all the three groups was comparable (p-value of 0.668). Gender distribution concerning all groups was done using the Chi-square test (p-value 0.795). Though there were more male than female patients in this study; all the groups had comparable demographic parameters (p-value >0.05) [Table/Fig-1]. A comparison of the type of surgeries performed on the study population concerning the three groups was done with the Chi-square test and found comparable (p-value of 0.999) [Table/Fig-2].
| Parameters | Group A (HES 200 group) (n=30) Mean±SD | Group B (HES 130 group) (n=30) Mean±SD | Group C (RL group) (n=29) Mean±SD | p-value |
|---|
| Mean age (years) | 37.06±11.34 | 35.21±10.71 | 38.03±12.19 | 0.587 |
| Mean weight (kg) | 65.55±7.54 | 64.62±8.9 | 63.65±9.24 | 0.668 |
| Male/Female | 17/13 | 20/10 | 19/10 | 0.795 |
| Type of surgeries | Group A (n=30) | Group B (n=30) | Group C (n=29) | p-value |
|---|
| General surgeries | 12 | 12 | 11 | 0.999 |
| Orthopaedic surgeries | 11 | 11 | 12 |
| Gynaecological surgeries | 7 | 7 | 6 |
The block height of T10-T6 was achieved in all the patients. Data showed the mean duration of surgeries were comparable (p-value of 0.224) [Table/Fig-3].
Mean duration of surgery.
| Patient group | Number of patients | Duration of surgery (in hours) | F-value | p-value |
|---|
| Mean | SD |
|---|
| Group A (HES 200) | 30 | 1.92 | 0.18 | 7.93 | 0.224 |
| Group B (HES 130) | 30 | 1.63 | 0.28 |
| Group C (RL) | 29 | 1.78 | 0.39 |
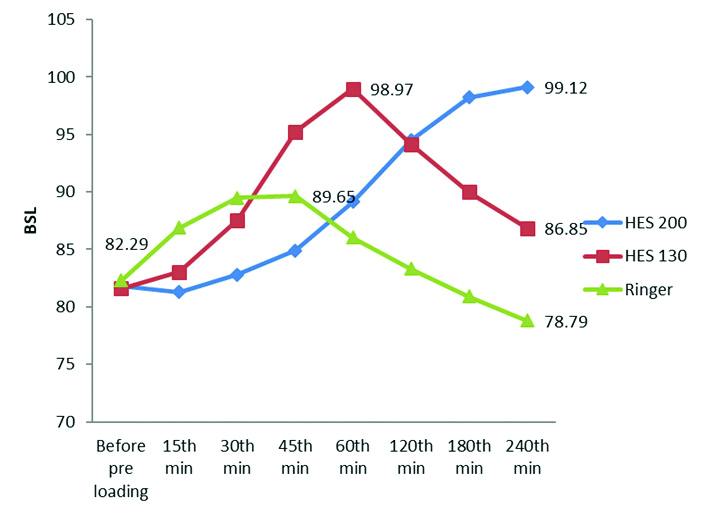
[Table/Fig-4] shows a comparison of changes in mean BSL in all three groups. The baseline BSL was comparable between the groups (p-value of 0.909).
| Time | Group A HES 200 (n=30) | Group B HES 130 (n=30) | Group C RL (n=29) | p-value |
|---|
| Mean±SD | Mean±SD | Mean±SD |
|---|
| Before co-loading | 81.85±6.34 | 81.62±7.14 | 82.29±5.94 | 0.909 |
| 15th min | 81.27±6.60 | 83.03±7.09 | 86.88±7.83 | 0.006 |
| 30th min | 82.82±6.11 | 87.56±7.88 | 89.50±8.15 | 0.001 |
| 45th min | 84.91±6.26 | 95.24±7.63 | 89.65±8.64 | <0.001 |
| 60th min | 89.12±6.73 | 98.97±10.88 | 86.03±7.13 | <0.001 |
| 120th min | 94.52±7.22 | 94.15±9.16 | 83.30±7.21 | <0.001 |
| 180th min | 98.24±7.55 | 90.00±7.59 | 80.91±6.34 | <0.001 |
| 240th min | 99.12±8.47 | 86.85±7.93 | 78.79±6.02 | <0.001 |
p-value less 0.001 was considered statistically significant
BSL changed from the baseline values (p-value of 0.006; ≤0.05) with an increase from 15 minutes onward (81.27±6.60 mg/dL in Group A; 83.03±7.09 mg/dL in Group B and 86.88±7.83 mg/dL in Group C). At 45th minutes, after administration of co-loading of fluid, this increase in BSL became highly significant (p-value is <0.001) with 84.91±6.26 mg/dL in Group A; 95.24±7.63 mg/dL in Group B and 89.65±8.64 mg/dL in Group C. Interestingly, this increase in BSL was more in the Group B i.e., in HES 130 group in comparison to the other two groups at 45th minutes. This highly significant increase continued until 240th minutes (p-value is <0.001) with a BSL of 99.12±8.47 mg/dL in Group A; 86.85±7.93 mg/dL in Group B and 78.79±6.02 mg/dL in Group C.
The same parameter is plotted in a graphic form in [Table/Fig-5] to understand the intra group analysis of variation of BSL following administration of co-loading fluid. The rise in mean BSL was at peak value at 60th minutes in Group B (HES 130) while the mean BSL continued to increase till 240th minutes in Group A (HES 200). The line diagram for the changes in BSL in Group C (RL group) showed a steep rise in the initial period reaching a peak value at 45th minutes which subsequently decreased in a graduated fashion below the baseline value after 120 minutes [Table/Fig-5].
Line diagram comparing mean blood sugar levels in all the three group.
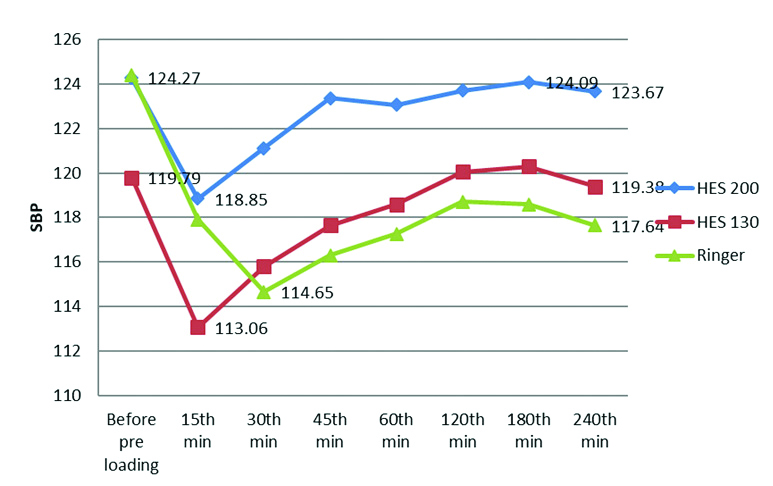
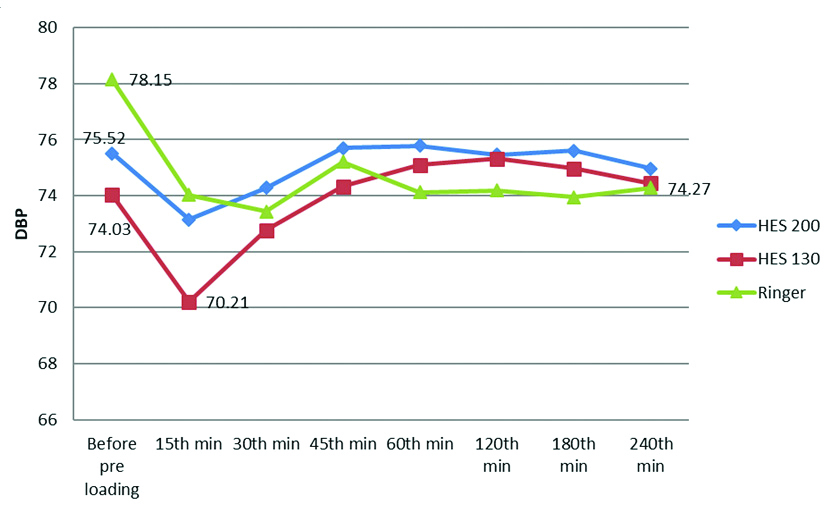
There was a statistically significant difference in SBP among the groups at 45th minute (p-value was 0.033) and at 240th minutes (p-value was 0.044). However, the variations in SBP were within physiological limits and clinically insignificant. There was no statistically significant change in the mean of the DBP in all the groups during the study [Table/Fig-6,7].
Line diagram comparing Systolic Blood Pressure (SBP) in all the three groups.
Line diagram comparing Diastolic Blood Pressure (DBP) in all the three groups.
Discussion
In the present study, there was an increase in BSL in all the three groups, which was attributable to the co-loading fluids given. BSL with RL group increased gradually but at a comparatively faster rate than the HES groups and peaks at 45th minutes and then gradually decreased till it fell below baseline value after 3 hours. The most plausible explanation for this would be the faster breakdown of lactate to glucose. Once the co-loading was over with RL, maintenance fluid was kept with plasmalyte. There was no further rise in BSL followed by a fall below the baseline value by the end of 240th minutes. Studies have been done to assess the effect of low-substituted high MW HES (HES 500/0.42 and HES 900/0.42) on coagulation profile comparing with low-substituted low MW HES (130/0.42) and concluded that there was no compromise on blood coagulation to a significant extent [11,12]. In a study conducted by Madjdpour C et al., suggested that MW may not represent a key factor in determining the effects on blood coagulation of HES [13]. In the present study; it was decided to find out the effect on BSL due to variations in the MS in the preparation of HES when used as a co-loading fluid. The BSL in the HES 130/0.4 group increased but more gradually than the RL group and peaked at 60th minutes. The increase in BSL in HES 200/0.5 group was still more gradual as compared to HES 130/0.4 and continued to rise till 240th minutes. This can be explained by the slower breakdown of HES to glucose by plasma α-amylase due to substitution at C2 and C6 position by the hydroxyethyl group. The slower increase and more sustained increase in BSL in HES 200/0.5 as compared to HES 130/0.4 may also be explained by comparatively higher MS, which makes the amylopectin more resistant to breakdown by plasma alpha-amylases [1].
In the present study, co-loading was done with 20 mL/kg body weight for the RL group (Group C) and 20 mL/kg body weight for both the HES groups (Group A and B). The volume of HES at a rate of 20 mL/Kg in the present study allows us to assess the effect on blood sugar (if any) at the maximum transfused volume. A study used a lower volume of HES when compared with RL [6]. In the present study, the haemodynamic variations in the pulse rate, SBP, DBP [Table/Fig-6,7] did not show any statistically significant differences between the groups when compared at several intervals of the study. This is in confirmation with an earlier study on haemodynamic effects of HES, i.e., the fixed amount of colloid exerts better or equivalent haemodynamic effect than the same amount crystalloid [14].
In an animal study, it was observed that HES infusions did not change blood glucose levels [15]. One explanation for that was the rapid urinary excretion of the smaller molecules of the product of HES metabolism and no further conversion to glucose formation [16]. Another explanation was initial rapid amylase-dependent break-down following HES infusion within the first 24 hour, 50% of which was excreted by the kidneys [17]. These might be the reasons why HES did not alter blood glucose levels significantly. The present study contradicts this explanation as it recorded a significant increase in BSL though within the physiological limit. Murty SS et al., also documented similar findings [3]. The present study advocates for the use of HES within its maximum dose limit but cannot comment on the use of HES in diabetic patients who were not part of the study population. In the study comparing the effects of 6% Hestarch-450, Pentastarch 200, and RL on BSL when used as preloading fluids under spinal anaesthesia, Murty SS et al., reached to the conclusion that both formulation of starch increases BSL significantly [3]. BSL reached to its peak value with Hestarch 6%-450 at the end of two hours whereas it was at the end of 3 hours for Pentastarch 6%-200. However, RL didn’t show significant effect. This finding was similar to the present study, though the results could not be extrapolated for comparison as the HES preparation used was different in MW and MS in that particular study.
Patki A and Selgaonkar VC in 2010 studied the effect of 6% HES -450, RL and low MW dextran on BSL during surgeries under SAB and concluded that, under stressful conditions, RL and 6% HES 450 significantly raise BSL but within physiological limits, whereas Dextran-40 raises BSL, well above the physiological limit [2]. Their study also demonstrated a significant rise in the BSL with the infusion of HES in comparison with RL. Their conclusion was similar to the findings of the present study, but a direct comparison of the results is not possible as they used 6% HES 450 while in the present study, 6% HES 130 and 6% HES 200 were used. Russ M et al., conducted a study on the effect of HES 130/0.4 on BSL as compared to 4% gelatin in a swine model with induced mixed academia. They concluded that volume support with HES caused an increase in BSL in the porcine model. Thus, additional control of blood glucose seems recommendable whenever using HES [8]. Conclusion about the impact of HES on BSL of that study matches with the observations of the present study.
Lou S et al., conducted a study on the impact of 6% HES 130/0.4 on BSL and compared it with 4% gelatin in patients undergoing open-heart surgery [9]. They concluded that starch-based colloid (HES130) had a different impact on intraoperative blood glucose than a gelatin-based colloid which contradicts the present study finding. Jung KT et al., also published a comparative study of the impact of HES130 with that of RL in non-diabetic patients undergoing surgeries under SAB [6]. They took BSL till six hours after administration of IV fluid and concluded that there is no significant difference between the groups. “Surviving Sepsis Campaign: International Guidelines for Management of Severe Sepsis and Septic Shock: 2016 and 2018” update had recommended against the use of HES for fluid resuscitation of severe sepsis and septic shock based on the results of the VISEP [18], CRYSTMAS [19], 6S [20], and CHEST trials [21]. The present study talks about the co-loading of fluid in surgeries under spinal anaesthesia and up to a limited volume. So, the findings of this study tried to figure out the effects of HES and its feasibility to become an alternative to the conventionally used fluid-like RL. In critically ill patient it is better to follow the recommendations as mentioned above. In 2013, the United States FDA issued a black box warning on the use of HES in critically ill patients. However, the European countries still use HES in major surgeries and trauma victims in the perioperative and intensive care settings due to its favourable haemodynamic effect [22].
Limitation(s)
The present study was conducted on ASA physical status I and II patients with no history of diabetes, and the result of the study cannot be extrapolated on the diabetic population. This was the main limitation of the study. Also, the increase in BSL continued in HES 200 group till the end of 240 minutes after which we did not measure BSL; and hence we cannot comment whether the peak level had been achieved or not. Adding to the limitation, common medications such as antibiotics, Non Steroidal Anti-Inflammatory Drugs (NSAIDs), and other drugs which may have influenced BSL were excluded from this study.
Conclusion(s)
HES in various preparations increases BSL in patients undergoing lower abdominal and lower limb surgeries under SAB, but this increase was within the physiological limits. Based on this study result, HES does not seem to pose a danger of hyperglycaemias, when used within the recommended dose as co-loading fluid for spinal anaesthesia in a non-diabetic patient.
p-value less 0.001 was considered statistically significant