Introduction
The SARS-CoV-2 belongs to the broad family of viruses known as β-coronavirus. It is a positive-sense single-stranded RNA (+ssRNA) virus, with a single linear RNA segment causing the Coronavirus Disease-2019 (COVID-19) disease. Each SARS-CoV-2 virion is 50-200 nanometres in diameter. Like other coronaviruses, SARS-CoV-2 has four structural proteins, known as the S (spike), E (envelope), M (membrane), and N (nucleo-capsid) proteins; the N protein holds the RNA genome, and the S, E, and M proteins together create the viral envelope. The spike protein, which has been imaged at the atomic level using cryogenic electron microscopy, is the protein responsible for allowing the virus to attach to and fuse with the membrane of a host cell; specifically, its S1 subunit catalyses attachment, the S2 subunit helps in fusion. It emerged in China in December 2019 and rapidly propagated in numerous countries, having contaminated more than 3,55,060 people, killing more than 11,922 people in India [1] and in the world infected numbers are 80,06,427 and deaths around 4,36,899 up to June 17, 2020 reported to the WHO [2]. The statistics are quite frightening and no specific medicine and prognostic markers are available yet.
Interferons in Viral Diseases
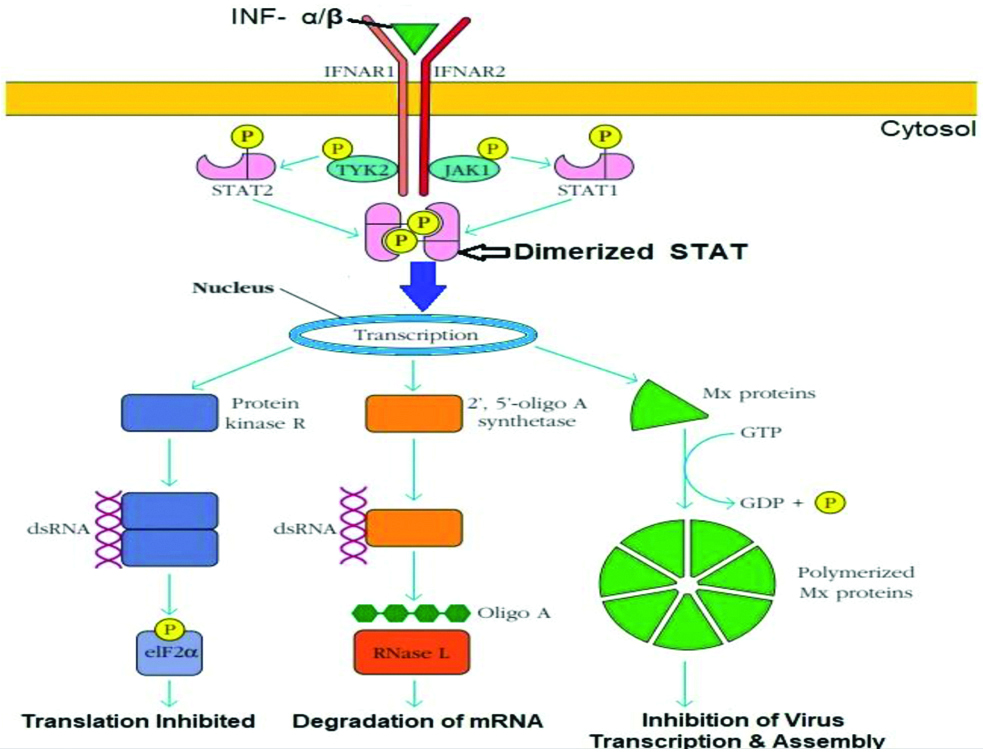
The IFNs are an extraordinary group of proteins with important effects on the immune system. Their actions affect both the adaptive and the innate arms of the immune system and include the induction of both class I and class II Major Histocompatibility Complex (MHC) molecules and augmentation of natural killer (NK)-cell activity. They are secreted by the cells of immune systems (WBCs, NK cells, fibroblasts and epithelial cells). Also, IFN can be manufactured using recombinant DNA technology. Three types of IFN are known till date. Type I IFN are composed of IFN-α (a family of about 20 related proteins) and IFN-β which are secreted by activated macrophages and dendritic cells as well as virus-infected cells after recognition of viral components by Pattern Recognition Receptors (PRRs) located either at the cell surface or inside the cell (endosome). The secreted type I IFN then interact in turn with membrane-bound IFN receptor and include the induction of ribonucleases that destroy viral (cellular) RNA and cessation of cellular protein synthesis thus, inhibiting viral replication. Simultaneously, they inhibit normal cellular function and destroy the virus infected cells so that infection cannot spread [1]. The IFN-α and IFN-β binds to the IFNAR receptor, which then recruit and activate the JAK1 and TYK2 protein kinases. They bind and phosphorylate STAT 1 and STAT 2, which dimerise, enter the nucleus, and stimulate expression of three proteins that activate antiviral effects. Protein Kinase R (PKR) binds viral dsDNA and inhibits the activity of the eIF 2 α translation initiation factor. A 2’, 5’-oligoadenylate synthase synthesises 2’, 5’-oligoadenylate, which activates a ribonuclease, RNase L, that degrades mRNAs. Mx proteins self-assemble into ring like structures that inhibit viral replication and formation of new virus particles [Table/Fig-1]. Type II IFN, otherwise known as IFN-γ is produced by activated T and NK cells and is released as a dimer. IFN-γ is a powerful modulator of adaptive immune response, biasing T-cell help towards the TH1 type and inducing the activation of macrophages, with subsequent destruction of any intracellular pathogens and the differentiation of cytotoxic T-cells. IFN-γ is used medically to bias the adaptive immune system toward a cytotoxic response so that it can destroy the infected cells. Type III IFN family was discovered in 2003. Three members of the family are IFN-λ1 (IL-29), IFN-λ2 (IL-28A) and IFN-λ3 (IL-28B). Like type I IFN, type III IFN also up regulates the expression of genes controlling viral replication and host cell proliferation [1].
Schematic diagram of major antiviral activities induced by Type I IFN.
Therapy with IFN
The IFN-α has been used for the treatment of hepatitis C, hepatitis B, hairy cell leukaemia, chronic myelogenous leukaemia (70% response rates have been reported in the chronic phases), and Kaposi’s sarcoma (associated with AIDS). IFN-β has emerged as the first drug capable of producing clinical improvement in multiple sclerosis. IFN-γ has been used with varying degree of success to treat Non-Hodgkin’s lymphoma, cutaneous T-cell lymphoma and multiple myeloma. A more successful clinical application of IFN-γ is in the treatment of the hereditary immunodeficiency Chronic Granulomatous Disease (CGD). Therapy of CGD patients with IFN-γ significantly reduces the incidence of infections. IFN-γ has also been shown to be effective in the treatment of osteopetrosis. Although IFN are powerful modifiers of biological responses, the side effects accompanying their use are fortunately mild. So, the use of IFN in clinical practice is likely to expand more [1]. IFN available as drugs are as follows:
IFN-α2a (Roferon-A)
IFN-α2b (Intron-A)
IFN-αn3 (Alferon-N)
PEG-IFN-α2b (PegIntron, Sylatron)
IFN-β 1a (Avonex, Rebif)
IFN-β1b (Betaseron, Extavia)
IFN-γ1b (Actimmune)
PEG-IFN-α2a (PegasysProClick)
PEG-IFN-α2a and ribavirin (Peginterferon)
PEG-IFN-β2b and ribavirin (PegIntron/Rebetol Combo Pack)
PEG-IFN-β1a (Plegridy)
IFNalfacon-1 (Infergen has been discontinued in the US) [2]
Scope of Interferons in COVID-19 Infections
It is clear from the general discussion about IFN that they are the most effective soldiers of innate immunity to fight against the viral infections and also commercially available IFN has been found to be very effective against hepatitis viruses, some cancers, autoimmune diseases and dreadful bacterial infections. Type 1 IFN have a broad antiviral activity invitro and have also been evaluated in a clinical trial to treat MERS-CoV [3]. In this regard, we are trying to evaluate the potential benefits of type 1 IFN as a promising and safer treatment modality as well as specific prognostic marker when there are no specific and established vaccines, drugs or prognostic biomarkers available yet against SARS-CoV-2.
Prognostic Aspects of Interferon Against COVID-19
It has been shown in a study by Eni Williams, that patients with SARS who had been discharged from hospital had low IFN-α and IFN-γ activity [4]. From previous discussion it is very evident that IFN-α and IFN-γ have a predominant antiviral activity in innate immunity both by inhibiting the viral replication (IFN-α and β) and killing the virus infected cells (IFN-γ).
Till date serum ferritin and C-Reactive Protein (CRP) are used as prognostic markers which are very non-specific and can be increased in various conditions like acute and chronic liver diseases, End Stage Renal Disease (ESRD), malignancies (leukaemia, Hodgkin’s disease), infections (viral and bacterial), inflammation (arthritis), acute myocardial infarction, iron storage disorders like haemosiderosis, idiopathic haemochromatosis, and almost all the broad types of anaemias like megaloblastic, haemolytic, sideroblastic, thalassaemia major and minor, spherocytosis, porphyria cutanea tarda etc., except iron deficiency anaemia [5]. Randomised control trials can be conducted among COVID-19 patients and their IFN levels can be assayed, along with serum ferritin and CRP. This can help to find out whether patients with serious illness have lower levels of IFN than those under recovery or with mild illness. Moreover regression analysis can be used to ascertain which parameters among these three have strong concordance with severity of COVID-19 illness. A positive outcome of the trial will enable the identification of a definite prognostic marker (serum IFN), which could be used to treat COVID-19 patients.
Therapeutic Aspects of Interferon Against COVID-19
Vaccines Against COVID-19 vs Interferon
A COVID-19 vaccine is a hypothetical vaccine against COVID-19. Several attempts to develop an effective vaccine have been made but none of them completed its course out of phases of clinical trials. As this RNA virus is very much mutation prone that imparts a huge problem in vaccine development [6,7]. By May, 120 vaccine candidates were in development, with five having entered Phase I-II clinical trials and six in Phase I trials [8,9]. Till date, no effective vaccine is available in the market.
The National Institutes of Health (NIH), USA have published interim guidelines for the medical management of COVID-19 prepared by the COVID-19 Treatment Guidelines Panel which includes antiviral therapy, chloroquine and hydroxychloroquine (HCQ) with or without azithromycin, convalescent plasma and immunoglobulin therapy [10].
Chloroquine, Hydroxychloroquine with or without Azithromycin vs Interferon
The NIH interim guidelines haven’t given approval to high dose chloroquine (i.e., 600 mg twice daily for 10 days) for the treatment of COVID-19 [10]. Firstly, high dose chloroquine is not generally given to children below 15 years and patients with cardiovascular ailments (as it prolongs QT interval) and retinal diseases. Even high dose carries a higher risk of toxicities than the lower dose. Some clinical trials have also been held worldwide, which indicate that it is not effective against COVID-19 as well as several other viruses. A study just published in a French medical journal reported some benefits of combination of HCQ and azithromycin for COVID-19 patients who had only mild symptoms of the virus. They also provide new evidence that HCQ does not appear to help the immune system to clear the coronavirus from the body. An observational study that has been published in the New England Journal of Medicine (NEJM) has emphasised the same by highlighting that HCQ is not actually reducing the numbers of death, especially in those patients who are the candidate for assisted breathing due to severe breathing difficulties and other comorbidities [11]. In this context, IFN could be evaluated in clinical trials to fast track the development of therapeutics against COVID-19. Having a wide range of antiviral activities, IFN is also very safe to administer in all groups of patients with several comorbidities. IFN-β 1b is already being evaluated in combination with ritonavir/lopinavir in the WHO’s “Solidarity” clinical trial for COVID-19 treatments [12]. Its detailed mechanism of action is described later.
Convalescent Plasma and Immunoglobulin vs Interferon
A few trials are being conducted on convalescent plasma therapy in severely ill COVID-19 patients. None of the trials have been completed yet. They have shown some benefits. But this treatment needs extensive expertise and vigorous monitoring. So, plasma therapy can be of restricted use only, considering the cost-effectiveness aspect of this treatment. On the other hand, treatment with IFN needs less vigorous monitoring and expertise and it is already commercially available in clinical practice. So, considering cost-effectiveness it should be given a try against SARS-CoV-2.
Interferon vs Antiviral Drugs
Antiviral treatments are warranted to contain the COVID-19 pandemic. Several candidates are already being investigated. In this context, IFN can also be evaluated as a treatment modality [13]. MERS-CoV and SARS-CoV-1 are coronaviruses closely linked with SARS-CoV-2 and present similar properties, despite some differences in their epidemiology, pathology and in several of their proteins [14]. IFN-I treatment has been studied against MERS-CoV and SARS-CoV-1 [15]. Innumerous invitro and invivo experiments, in combination with lopinavir/ritonavir [16,17], ribavirin [18-20], remdesivir, corticosteroids [21] and IFN-γ [22,23] class 1 IFN have been shown to be effective. Consequently, IFN-β1 seems to be more pertinent IFN to treat coronavirus infections. This can be associated with the protective activity of IFN-β1 in the lungs: it up regulates cluster of differentiation factor 73 (CD73) in pulmonary endothelial cells, resulting in secretion of anti-inflammatory adenosine, helping in maintenance of endothelial barrier. That explains why treatment with IFN-β1 shows reduction of vascular escape in Acute Respiratory Distress Syndrome (ARDS) [24]. The combination of IFN-β1b (injected intravenously) and lopinavir/ritonavir has been investigated in a clinical trial in Saudi Arabia [25]. This is to our knowledge the only clinical trial against MERS-CoV.
“Solidarity” clinical trial for COVID-19 treatment is a multinational phase-III-IV clinical trial announced by WHO on 18th March, 2020. The trial concentrates on certain treatment modalities against COVID-19. One of them is evaluation of IFN-β1b along with ritonavir/lopinavir in adults (age ≥18 years) recently hospitalised, or already in hospital, with confirmed COVID-19 and, in the view of the responsible doctor [12].
Mechanism of Action of Type 1 IFN against SARS-CoV-2
Previous successful clinical trials with IFN-1 against SARS-CoV-1 and MERS-CoV can give some insight on its potentials of being selected as a valued treatment option against SARS-CoV-2. The Orf6 and Orf3b proteins of SARS-CoV-2 are truncated [26] and may have lost their anti-IFN functions. It could explain why SARS-CoV-2 displays invitro a substantial sensitivity to IFN-α [26]. Although SARS-CoV-2 replication is not entirely suppressed by IFN, viral titres are decreased by several orders of magnitude. SARS-CoV-2 is found to be more sensitive to IFN-1 than SARS-CoV-1. Even IFN-α 2b sprays can reduce the infection rate of SARS-CoV-2 [27]. From above discussion it is very much evident that IFN-I treatment should be more effective for the recent evil i.e., SARS-CoV-2 than its previous known congener. Even in India a three-member team at HCG Hospital, Bengaluru has developed a treatment protocol for COVID-19 positive patients. The team is of the view that IFN can be administered to the patient at the early stages of the disease to reduce the morbidity and severity of pulmonary complications [28,29].
Conclusion(s)
To conclude, a randomised control trial among the patients classified by their severity can be performed to ascertain whether IFN can be established as a specific prognostic marker or not. Moreover, type 1 IFN may be a safe and easy modality to upscale treatment against COVID-19, since its safety has already been assessed in numerous independent clinical trials. More extensive studies are needed on an urgent basis for its judicious use to combat SARS-CoV-2.
[1]. Covid 19 India A crowd sourced initiative [Internet]. Covid 19 India; 2020 [updated 2020 June 16; cited 2020 June 18] [Google Scholar]
[2]. WHO Coronavirus Disease (COVID-19) Dashboard [Internet]. World health organization; 2020 [updated 2020 June 17; cited 2020 June 18] [Google Scholar]
[3]. Owen, Punt, Stranford. Kuby Immunology, 7th edition. Macmillan higher education International edition, 2013;119-21:165 [Google Scholar]
[4]. Eni Williams. Interferon: Potential COVID-19 Treatment [Internet]. MedicineNet. 2020 [updated 2020 March 15; cited 2020 May 2] [Google Scholar]
[5]. Sallard E, Lescure FX, Yazdanpanah Y, Mentre F, Peiffer-Smadja N, Florence AD, Type 1 interferons as a potential treatment against COVID-19Antiviral Research 2020 2020:10479110.1016/j.antiviral.2020.10479132275914 [Google Scholar] [CrossRef] [PubMed]
[6]. Cameron MJ, Ran L, Xu L, Danesh A, Bermejo-Martin JF, Cameron CM, Interferon-mediated immunopathological events are associated with atypical innate and adaptive immune responses in patients with severe acute respiratory syndromeJournal of Virology 2007 81(16):8692-706.10.1128/JVI.00527-0717537853 [Google Scholar] [CrossRef] [PubMed]
[7]. Williamson MA, Snyder LM, Wallach’s Interpretation of Diagnostic Tests Pathways to Arriving at a Clinical Diagnosis-e-book 2015 10th editionWolters Kluwer [Google Scholar]
[8]. Wiedermann U, Garner-Spitzer E, Wagner A, Primary vaccine failure to routine vaccines: why and what to do?Human Vaccines & Immunotherapeutics 2016 12(1):239-43.10.1080/21645515.2015.109326326836329 [Google Scholar] [CrossRef] [PubMed]
[9]. Eyal N, Lipsitch M, Smith PG, Human challenge studies to accelerate coronavirus vaccine licensureThe Journal of Infectious Diseases 2020 221(11):1752-56.10.1093/infdis/jiaa15232232474 [Google Scholar] [CrossRef] [PubMed]
[10]. Le TT, Andreadakis Z, Kumar A, Roman RG, Tollefsen S, Saville M, The COVID-19 vaccine development landscapeNat Rev Drug Discov 2020 19(5):305-06.10.1038/d41573-020-00073-532273591 [Google Scholar] [CrossRef] [PubMed]
[11]. Geleris J, Sun Y, Platt J, Zucker J, Baldwin M, Hripcsak G, Observational Study of Hydroxychloroquine in Hospitalized Patients with Covid-19N Engl J Med 2020 382:2411-18.10.1056/NEJMoa201241032379955 [Google Scholar] [CrossRef] [PubMed]
[12]. World Health Organization. Accelerating a safe and effective COVID-19 vaccine [Internet]. World health organization; 2020 [updated 2020 April 27; cited 2020 May 02] [Google Scholar]
[13]. National Institutes of Health. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines [Internet]. National Institutes of Health; 2020 [updated 2020 July 17; cited 2020 July 25] [Google Scholar]
[14]. World health organization. “Solidarity” clinical trial for COVID-19 treatments [Internet]. World health organization; 2020 [updated 2020 July 06; cited 2020 July 25] [Google Scholar]
[15]. Martinez MA, Compounds with therapeutic potential against novel respiratory 2019 coronavirusAntimicrob Agents Chemother 2020 64(5):e00399-20.10.1128/AAC.00399-20 [Google Scholar] [CrossRef]
[16]. Lai CC, Shih TP, Ko WC, Tang HJ, Hsueh PR, Severe acute respiratory syndromecoronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): the epidemicand the challengesInt J Antimicrob Agents 2020 55:10592410.1016/j.ijantimicag.2020.10592432081636 [Google Scholar] [CrossRef] [PubMed]
[17]. Stockman LJ, Bellamy R, Garner P, SARS: Systematic review of treatment effectsPLoSMed 2006 3:1525-31.10.1371/journal.pmed.003034316968120 [Google Scholar] [CrossRef] [PubMed]
[18]. Sheahan TP, Sims AC, Leist SR, Schäfer A, Won J, Brown AJ, Comparative therapeutic efficacy of remdesivir and combination lopinavir, ritonavir, and interferon beta against MERS-CoVNat Commun 2020 11:22210.1038/s41467-019-13940-631924756 [Google Scholar] [CrossRef] [PubMed]
[19]. Chan JFW, Yao Y, Yeung ML, Deng W, Bao L, Jia L, Treatment with lopinavir/ritonavir or interferon-β1b improves outcome of MERS-CoV infection in an on human primate model of common marmosetJ Infect Dis 2015 212:1904-13.10.1093/infdis/jiv39226198719 [Google Scholar] [CrossRef] [PubMed]
[20]. Chen F, Chan KH, Jiang Y, Kao RYT, Lu HT, Fan KW, In vitro susceptibility of 10 clinical isolates of SARS coronavirus to selected antiviral compoundsJ Clin Virol 2004 31:69-75.10.1016/j.jcv.2004.03.00315288617 [Google Scholar] [CrossRef] [PubMed]
[21]. Morgenstern B, Michaelis M, Baer PC, Doerr HW, Cinat lJ, Ribavirin and interferon-βsynergistically inhibit SARS-associated coronavirus replication in animal and human celllinesBiochem Biophys Res Commun 2005 326:905-08.10.1016/j.bbrc.2004.11.12815607755 [Google Scholar] [CrossRef] [PubMed]
[22]. Omrani AS, Saad MM, Baig K, Bahloul A, Abdul-Matin M, Alaidaroos AY, Ribavirin and interferon alfa-2afor severe Middle East respiratory syndrome coronavirus infection: A retrospective cohortstudyLancet Infect Dis 2014 14:1090-95.10.1016/S1473-3099(14)70920-X [Google Scholar] [CrossRef]
[23]. Loutfy MR, Blatt LM, Siminovitch KA, Ward S, Wolff B, Lho H, Interferonalfacon-1 plus corticosteroids in severe acute respiratory syndrome: A preliminary studyJ Am Med Assoc 2003 290:3222-28.10.1001/jama.290.24.322214693875 [Google Scholar] [CrossRef] [PubMed]
[24]. Sainz B, Mossel EC, Peters CJ, Garry RF, Interferon-beta and interferon-gammasynergistically inhibit the replication of severe acute respiratory syndrome-associated coronavirus (SARS-CoV)Virology 2004 329:11-17.10.1016/j.virol.2004.08.01115476870 [Google Scholar] [CrossRef] [PubMed]
[25]. Scagnolari C, Vicenzi E, Bellomi F, Stillitano MG, Pinna D, Poli G, Increased sensitivity of SARS-coronavirus to a combination of human type I and type II interferonsAntivir Ther 2004 9(6):1003-11. [Google Scholar]
[26]. Bellingan G, Maksimow M, Howell DC, Stotz M, Beale R, Beatty M, The effect of intravenous interferon-beta-1a (FP-1201) on lung CD73 expression and on acute respiratory distress syndrome mortality: an open-label studyLancet Respir Med 2014 2:98-107.10.1016/S2213-2600(13)70259-5 [Google Scholar] [CrossRef]
[27]. Arabi YM, Alothman A, Balkhy HH, Al Dawood A, Al Johani S, Al Harbi S, Treatment of Middle East Respiratory Syndrome with a combination of lopinavir-ritonavir and interferon-β1b (MIRACLE trial): Study protocol for a randomised controlled trialTrials 2018 19:01-13.10.1186/s13063-017-2427-029382391 [Google Scholar] [CrossRef] [PubMed]
[28]. Lokugamage KG, Hage A, Schindewolf C, Rajsbaum R, Menachery VD, SARS-CoV-2 is sensitive to type I interferon pretreatmentBioRxiv 2020 Jan 1 10.1101/2020.03.07.982264 [Google Scholar] [CrossRef]
[29]. Balasubramanyam KR, COVID-19: HCG team’s find offers hope for treatmentThe Economic Times: Healthcare 2020 Mar 31 [cited 2020 May 15] [Google Scholar]