Introduction
COVID-19 is a zoonotic virus and from phylogenetic analyses, bats found to be the reservoir of COVID-19 virus as of now, but it has not yet been identified that what are the intermediate host(s) [1]. Coronavirus came into the highlight in 2002-2003 when clumps of “atypical pneumonia” were first reported in Guangdong Province, followed by spreading to Hong Kong. Coronavirus is viruses of the family Coronaviridae and is a class of genetic diverse viruses, which is a single strand, positive-sense RNA genome ranging from 26 to 32 kilobases in length and has been identified in various avian hosts as well as in various mammalian species, including camels, bats, dogs, and cats [2-4].
Another virus of the family Coronaviridae is the SARS and COVID-19 is worse than SARS because the virus spreads before symptoms appear. SARS is the first new infectious disease of this decade and first originated in 2002 end from Southern China with a high mortality and morbidity and also affected 8,000 people and more with nearly 800 deaths [5]. SARS is caused by a novel SARS-CoV, and initially SARS cases were reported from a hospital in Hanoi, Vietnam, by Carlo Urbani, who was a WHO scientist and himself died from the disease [6-9]. Many coronaviruses may cause intestinal and respiratory infections in animals and humans.
The other member of the coronavirus family is Middle East Respiratory Syndrome (MERS)-CoV, which is transmitted from dromedary camels to a man in Saudi Arabia in a private hospital in Jeddah on June 13, 2012, with history of fever, cough, expectoration, and shortness of breath [10]. Later the virus first named as 2019-nCoV after isolated from human patients and molecular analysis showed that the pathogen was a new coronavirus, and subsequently renamed as COVID-19 by WHO [11]. This outbreak seemed to be linked with a large seafood and animal market, and investigations are still ongoing to determine its origin [12].
The present and highly infectious outbreak of respiratory tract infections, including Respiratory Distress Syndrome (RDS), is the third spill over, in only two decades, of an animal coronavirus infection to humans after mutation, resulting in a major epidemic and the incubation period (time from exposure to the development of symptoms) of the COVID-19 is estimated to be between 2 and 14 days [13,14]. The CSG certified this virus as a sister of the SARS-CoVs species and named as SARS-CoV-2.
Infection of SARS-CoV-2 activates innate and adaptive immune response of human body in a rapid and well-coordinated immune response which represents the first line of defence against the viral infection, and the host immune defence may cause harmful tissue damage at both at the site of virus entry and at systemic level. As the excessive proinflammatory host response has been hypothesised to induce the immune pathway resulting in the rapid course of acute lung injury in SARS-CoV-2 infected patients, and after injury there is an immense cytokine and chemokine discharge called “cytokine storm”, that clearly reflects a widespread uncontrolled dysregulation of host immune defence. That’s why the key role of the immune system in SARS-CoV-2 infected patients might give us clues for the clinical management of the severe cases and for preventing the transition from mild to severe stages and in this review authors will describe, how the immune system plays the first line of defence against viral infection.
Humans may be infected by and suffer clinical consequences from different type of viruses, and in most the cases the infection is resolved with or without damage of the infected persons. In this review, we discuss our current understanding of the circumstances of SARS CoV-2 infection and will also discuss about host immune interaction against COVID-19.
Some Facts about Coronaviruses
SARS-CoV-2 mainly targets airway epithelial cells, alveolar epithelial cells, vascular endothelial cells and macrophages in the lung, all of which express the Angiotensin-Converting Enzyme 2 (ACE2).
What We Know and What We Don’t
According to Marion Koopmans Erasmus MC, the article published in cell press Leading Edge Voices, this outbreak is speedily becoming the first true pandemic challenge that fits the disease X category whether it will be checked or not and it is recorded to the WHO’s priority list of diseases for which we need to be prepared. Its origin, the diseases associated with infection, and the ability to spread are clear on the basis of its initial resemblances with the SARS outbreak. The efforts that are needed to contain the epidemic are scaring because the global air travel has increased more than 10-fold since 2003. But unluckily, as in the past outbreaks, fundamental knowledge gaps and medical measures need to be assessed on the fly, and scientists and public health experts people alike are wasting valuable time in lettering grant applications to do what we know wants to be done but which is not an element of a regular venture in science and (global) public health awareness [15].
Genomic Organisation of SARS CoV-2 [
7]
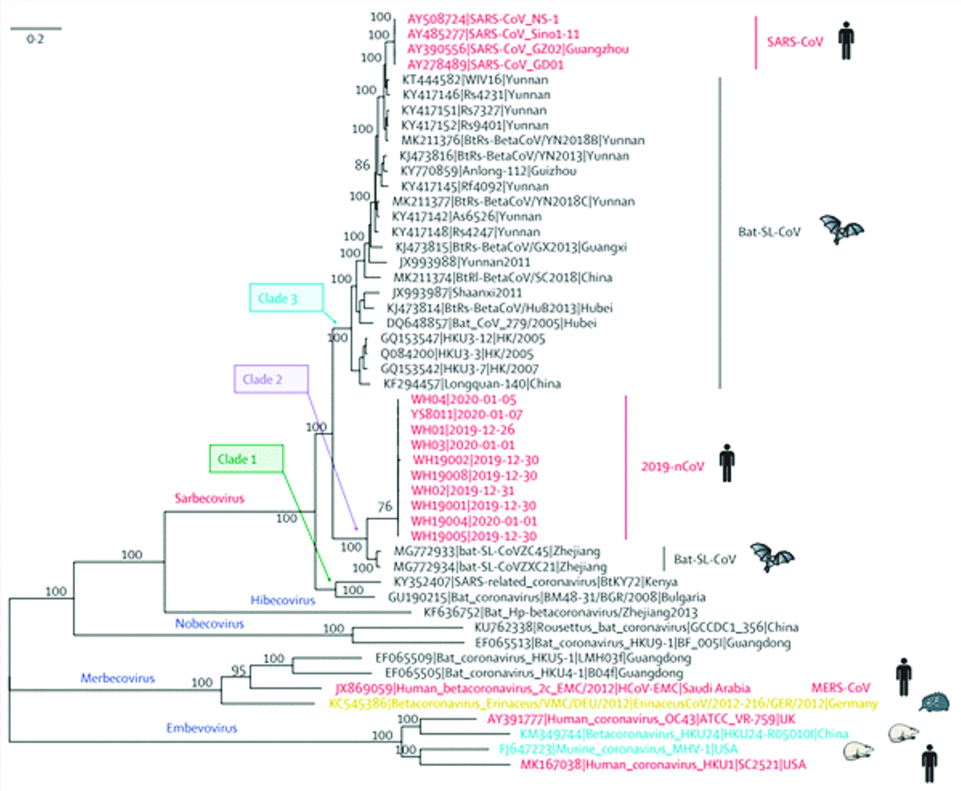
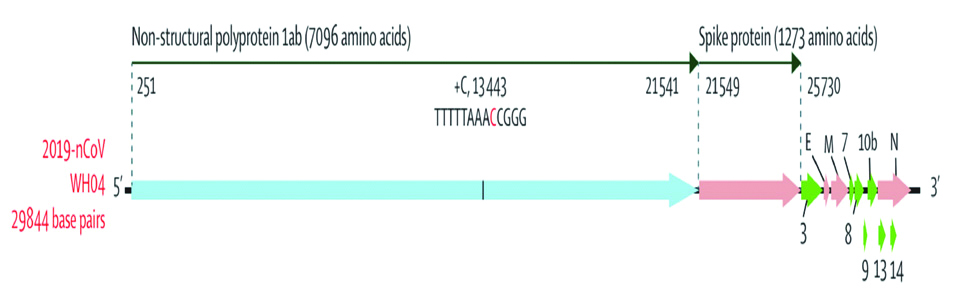
Alignment of COVID-19 genome sequencing including other available genomes of Betacoronavirus showed closest relationship with the bat SARS-like coronavirus (strain Bat Cov RaTG13) with 96% identity [1]. Phylogenetic analysis of 2019-nCoV and its closely associated mentioned genomes revealed that the five subgenera formed five well supported branches given in [Table/Fig-1]. The Sarbecovirus could be classified into three well supported clades (biological group). Clade 1 [two SARS-CoV-related strains from Rhinolophus sp. from Bulgaria (accession number GU190215) and Kenya (KY352407)]; Clade 2 [10 2019-nCoV from Wuhan and the two bat-derived SARS-like strains from Zhoushan in eastern China (bat-SL-CoVZC45 andbat-SL-CoVZXC21)]; and Clade 3 [SARS-CoV strains from humans and many genetically similar SARS-like coronaviruses from bats collected from southwestern China] [16]. The 5’UTR and 3’UTR are involved in inter and intra-molecular interactions and are functionally important for RNA-RNA interactions and for binding of viral and the cellular proteins. At 5’ end, 1ab is the first ORF of the whole genome length encoding non-structural proteins with size of 29844 bp (7096aa) in COVID-19. SARS-CoV-2 (COVID-19) binds to ACE2 (the angiotensin-converting enzyme 2) by its Spike and allows COVID-19 to enter and infect cells [Table/Fig-2].
Phylogenetic analysis of full-length genomes of 2019-nCoV and representative viruses of the genus Betacoronavirus. 2019-nCoV=2019 novel coronavirus. MERS-CoV=Middle East respiratory syndrome coronavirus. SARS-CoV=severe acute respiratory syndrome coronavirus [7].
Coding regions of 2019-nCoV only open reading frames of more than 100 nucleotides are shown. 2019-nCoV=2019 novel coronavirus.
Clinical Characteristics of COVID-19
Following are the main symptoms found in COVID-19 patients and it was based on the published literature [17-21].
80% of infections are mild i.e., with flu-like symptoms and can recover at home.
14% cases are severe, can develop into pneumonia and shortness of breath.
4.7% cases as critical and can include: respiratory failure, septic shock, and multi-organ failure require hospital treatment.
Near about 2% of reported cases, the virus is fatal.
Risk of death increases with age or with associated co-morbidities such as kidney disease, hypertension, diabetics.
Relatively few cases are seen among children.
Death rate is different and according to age, is given below in the [Table/Fig-3]. Difference between different type of flu and its symptoms is shown in [Table/Fig-4].
Death rate among patients.
| Age (years) | Death rate |
|---|
| 0-19 | 0.02% |
| 20 to 29 | 0.09% |
| 30 to 39 | 0.18% |
| 40 to 49 | 0.40% |
| 50 to 59 | 1.3% |
| 60 to 69 | 4.6% |
| 70 to 79 | 9.8% |
| 80 and above | 18% |
Note: a) 3.4% is the overall mortality rate estimate by the WHO as of March 3, 2020.
b) 15% is the death rate among hospitalised patients Hospital Fatality Rate (HFR) [14,19].
Different type of flu and its symptoms.
| Type fo flu | Symptoms |
|---|
| Air Pollution | Dry cough + Sneeze |
| Common Cold | Cough + Mucus + Sneeze + Runny nose |
| Flu | Cough + Mucus + Sneeze + Runny nose + Body ache + Weakness + Light fever |
| Coronavirus | Dry cough + Sneeze + Body pain + Weakness + High fever + Difficulty breathing |
Clinical Management of COVID-19
COVID-19 has asymptomatic, mild and severe infection, in which asymptomatic and mild infection may resolve without medical care and patients will be able to manage their illness at home being quarantined, but may progress to pneumonia and respiratory failure which require hospitalisation. Some patients of COVID-19 presented with severe disease requiring hospitalisation and required supportive management of the most common complications such as pneumonia, hypoxemic respiratory failure, sepsis, cardiomyopathy and arrhythmia, and complications from sustained hospitalisation, including secondary bacterial infections, gastrointestinal bleeding, and others critical illness [13,22-25]. Clinical management is totally dependent upon the symptoms of the infected person. Older men and men with co-morbidities such as hypertension, kidney disease, lung disease and diabetes are at high risk for severe disease, should be monitored closely given the possible risk of progression to severe illness in the second week after symptom onset [14,19,26,27]. For respiratory care, there is standard therapy, which is being used cautiously in COVID-19, and these include the use of high-flow nasal oxygen, non-invasive positive-pressure ventilation, nebulised medications, airway clearance therapies, all with the aim of minimising the risk of aerosolisation of the virus and airborne spread. Some drugs and vaccines are under trial, but till date no antiviral treatment for coronavirus infection has been proven to be effective and will discuss it detailed in next part of the review.
Prevention of COVID-19 Transmission
The prevention of this disease is important for the control and break down of the transmission of virus by manage and control those who are infected and still effecting. For this, preventive measures like wearing masks, avoid people gathering, and hand hygiene are also very important to break the chain of transmission [26]. In a large population country like India, there is much chance of cross-infection because one family may have several members. For confirmed cases, the hospital should have a good quarantine and isolation place to avoid cross-infection and break down the transmission. In addition, doctors should pay more attention for finding severe cases early in order to diagnose and treat critical cases and care to reduce the risk of mortality [26].
Body’s Immune System
The body’s first line of defence is the innate immune system that initiates right after an infection, and the infection may be virus, bacteria or any other antigens, and that innate immune system kills the virus and any cells damaged by it. The body’s second line of defence is the adaptive immune system, kicks in days later if any virus remains, employing what it has learned about the virus to mobilise a form of special forces such as T-cells and B-cells. B cells secrete antibodies that are proteins called immunoglobulins and they circulates in the bloodstream and permeate the other body fluids. Antibody bind specifically to the invading virus that stimulated their production. Antibody binding mark and targets invading pathogens for death by making it easier for phagocytic cells of the innate immune system to ingest them [7].
Immune Response to SARS-CoV-2
Immune system T-cells are called as immune warriors, but the role of T-cells in fighting against SARS-CoV-2, remains unclear. Against previous and new infection Naïve T-cells are useful as they release cytokines, whereas memory T-cells mediate antigen specific immune response. In a retrospective study done on 452 patients with COVID-19, by Qin C et al., the patients with severe COVID-19 exhibited a significantly lower number of both helper T-cells and suppressor T-cells [28], particularly among the helper T-cells, and a decrease in the regulatory T-cells, with a more marked reduction depending on the severity of the cases, and also decrease in the memory T-cells was noted, whereas the percentage of the naïve T-cells was found increased [29].
Several studies have found the relevant changes in innate and adaptive immune system in COVID-19 patients, especially in the lymphocytopenia and a modulation in total neutrophils are the common authentications and appear to be the direct correlation with severity of disease and death [30,31]. In case of severe COVID-19, the number of circulating CD4+ cells, CD8+ cells, B-cells and Natural Killers (NK) cells and eosinophils (monocytes and basophils) are decreases [31] and significantly increased serum levels of proinflammatory cytokines (e.g., Interleukin (IL)-6, IL-1β, IL-2, IL-8, IL-17, Granulocyte Colony-Stimulating Factor (G-CSF), Granulocyte Macrophage Colony Stimulating Factor (GM-CSF), 10 kD Interferon Gamma-Induced Protein (IP-10), Monocyte Chemoattractant Protein-1 (MCP-1), CCL3 (Macrophage inflammatory protein 1-α (MIP 1-α) also known as CCL3), and Tumour Necrosis Factor (TNF) has been reported [8,28,32]. But no direct evidence for pro-inflammatory cytokines and chemokines involvement in the lung pathology in COVID-19 infected patient has been reported.
Interferons (IFN) are made up of proteins and release by the cells in response to the virus infection, and interferons are categorised into 3 categories: named as type I, type II, and type III. Insufficient and Incompatible activation of IFN signalling may be the reason of severe cases of COVID-19. Too less IFN at the starting and too much later during infection could also contribute to severe or even lethal cases. Specific subtypes of IFN such as IFN-α, IFN-β, IFN-γ used to treat chronic viral infections, such as viral hepatitis, autoimmune diseases such as multiple sclerosis, to treat a bone disorder and an immune deficiency syndrome. IFNs has lots of clinical applications in the treatment of viral diseases and IFN-α can be effective against SARS CoV-2. Mild symptoms (like colds) by any virus stimulate a strong IFN response and that’s why the patient can get recovered from virus after near about one week without any treatment.
Body’s Innate and Adaptive Immunity against SARS-CoV-2
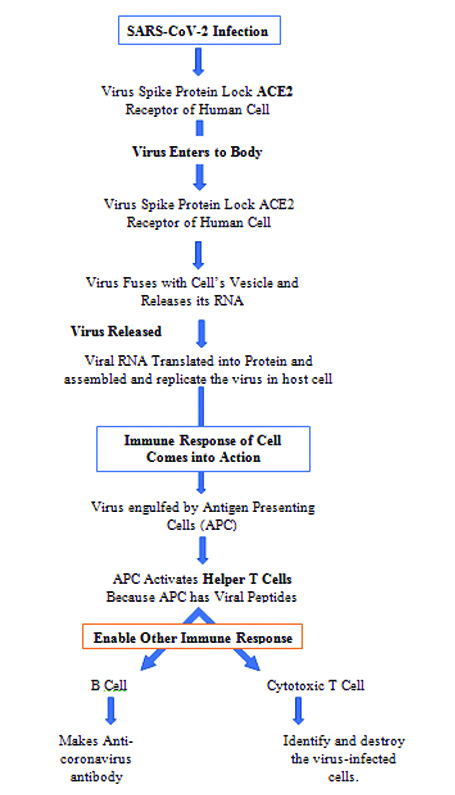
The ability of the immune system is to differentiate, what is foreign from what is self in this way is a fundamental characteristic of the adaptive immune system. The adaptive immune system is carried out by white blood cells called lymphocytes. The function of the adaptive immune system is to destroy the invading viruses, bacteria and any toxic molecules they produce. There are two broad categories of adaptive immune responses, first one is antibody responses and the second one is cell-mediated immune responses, and this immune response is carried out by different classes of lymphocytes, called as B-cells and T-cells, respectively. First line of defence against virus infection is Innate immunity and the receptors are activated against the viral infection that recognise the pathogen associated molecular patterns, such as double-stranded RNA or mRNA. The identification of pathogen associated with molecular patterns results in concomitant cytolytic immune responses, mainly through the type I IFN and NK cells. SARS-CoV-2 infection and the destruction of lung cells such as epithelial cells, and macrophages activates a local immune reaction, after infection macrophages and monocytes release T-cell, B-cell and cytokines immune reactions [7]. Both T and B-cell are detected in the blood about 1 week after the onset of COVID-19 symptoms [7]. CD4+ T-cells are essential to prime both CD8+ T-cells and B cells, whereas CD8+ T-cells are necessary for directly attacking and killing virus-infected cells. CD4+ T-cells are responsible for cytokine production for immune cell recruitment [8]. These CD4+ T cells contribute numerous activities in protective immunity against viruses that are initiated by infection or by vaccination. In most of the cases, this process is capable of dissolving the infection. But, in some cases, dysfunction of the immune response occurs, which can cause severe lung and even systemic pathology. The immunity development against a pathogen through natural infection is a multi-step process that generally takes place over 12 weeks. SARS-CoV-2 infection occur by binding to a host cell through its target receptor i.e., ACE2, it is because loss of pulmonary ACE2 function is associated with acute lung injury [32] and the destruction of lung cells triggers a local immune response, recruiting macrophages and monocytes that respond to the infection, release cytokines and adaptive T and B-cell immune responses. But in most of the cases, this process is able of resolving the infection, and in some of the cases, a dysfunctional immune response occurs, which can cause severe lung and even systemic pathology.
It is well known that COVID-19, which targets surface cells of the upper and lower respiratory system, and has an average incubation period of 6 to 14 days and a much slower disease progression. Presently, limited study and data is available on the host innate immune interaction of SARS-CoV-2 infected patients, but mathematical modelling indicates that the adaptive immune response may kick in before the target cells are depleted, slow down the infection rate and interfering with the innate immune response ability to eradicate most of the viruses quickly [33,34]. As the infection keeps persisting, it will circulate the whole of the adaptive immune response with its multiple layers. The fundamental interaction of the innate and the adaptive immune reactions may also explain why some COVID-19 patient’s experience two waves of the disease, and appearing to get better before eventually getting much worse.
SARS-CoV-2 is a Cytopathic virus, can cause death and injury of virus-infected cells and tissues as part of the virus replicative cycle, cause high levels of virus-linked pyroptosis, and is a highly inflammatory form of programmed cell death that is usually seen with cytopathic viruses [34,35]. This inflammation cause secretion of the pro-inflammatory cytokines and the chemokines IL-6, IFNγ, MCP1 and IP-10 into the blood of impaired patients and these cytokines are indicators of a T Helper 1 (TH1) cell [Table/Fig-5] [5,6].
Flow chart of virus replicative cycle, cause high levels of virus-linked pyroptosis, and is a form of programmed cell death.
The Antiviral, Synergistic, Immunomodulatory Effect of Drugs on Immune System against SARS-COV-2
If the virus continues to mutate to lower its pathogenicity, there is a high possibility that it might coexist with humans and it is possible after the development of a therapy to treat SARS-CoV-2 or vaccine to immunise. Because of the importance of immune instability in the pathogenesis of SARS-CoV-2 infection, there are several immune- regulating drugs that regulate different aspects of inflammation are being tested for their efficacy in the treatment of COVID-19. There are few key receptors and inflammatory cytokine which play a critical role in biological immuno-modulating drugs [Table/Fig-6] [36-48].
Antiviral, synergistic, immunomodulatory effect of drugs on immune system against SARS-CoV-2 [36-48].
| Drugs | Mode of action | Reference |
|---|
| Hydroxychloroquine or Chloroquine | Interference with ACE2 receptor to block virus invasion; increase of endosomal pH required for virus fusion; mild immune suppression | [36-39] |
| Thalidomide | Reduction of inflammatory cell infiltration; reduction of cytokine storm; reduction of lung damage | [40-43] |
| Tocilizumab (a recombinant humanised monoclonal antibody) | Blockade of IL-6 receptor and its downstream signalling pathways | [36,44] |
| Anakinra | Blockade of IL-1 receptor and its downstream signalling pathways | [19,45] |
| Ruxolitinib | JAK inhibitor; immune suppression | [46-48] |
JAK: Janus kinase
There are also some other treatment which is being started to treat COVID-19 patients such as stem cell therapy and plasma therapy [Table/Fig-7] [49-53].
Therapies and their mode of actions to treat COVID-19 patients.
| Therapy | Mode of action |
|---|
| Stem cell therapy | Suppression of inflammation; proviral silencing |
| Convalescent plasma | Promotion of virus elimination via virus-specific antibodies |
To recover the patient from COVID-19, Anti-viral immunity is mandatory. There are pros and cons of using medications on COVID-19 patients. They should be carefully considered as immune cell subsets and the evaluation of cytokine profiles has crucial significance for selecting appropriate treatment. For example tocilizumab could be considered in patients with high concentrations of serum IL-6. Also, the timing of treatment is very essential to repress the side-effects of immunosuppressants; but unfortunately, there is not yet any crucial evidence with regard to the suitable timing of administration of these agents. Therefore, studies are required to overcome this problem.
Drug under Clinical Trial against COVID-19
In the National Health Commission (NHC) guidelines, some drugs such as arbidol and chloroquine phosphate is a potent treatment options for COVID-19 patients and was shown to inhibit multiple enveloped viruses by inhibiting their entry/fusion of viral membranes with cellular membranes [54,55]. Chloroquine which is a traditional antimalarial drug, effective against SARS-CoV-2 infection invitro [56] and also several clinical trials are in progress for the efficacy and safety of chloroquine phosphate against COVID-19 [57].
Several antiviral drugs have been under clinical trial against viral proteases, polymerases, MTases, and entry proteins are given below in [Table/Fig-8].
Antiviral drugs under clinical trial against virus.
| Drug under clinical trial | Registration number |
|---|
| Remdesivir | NCT04252664 and NCT04257656 |
| Favipiravir | ChiCTR2000029544 and ChiCTR2000029600 |
| ASC09 | ChiCTR2000029603 |
| Lopinavir/Ritonavir | ChiCTR2000029387, ChiCTR2000029468, and ChiCTR2000029539 |
Remdesivir is a monophosphoramidate prodrug of an adenosine analog, and is an active form that can integrate into incipient viral RNA by the activity of RNA-dependent RNA polymerases, which then causes RNA synthesis arrest and thus virus replication is blocked.
Some clinical trial is summarised as shown below in [Table/Fig-9]. In addition, many more drug trial is ongoing.
Several other drugs under trial.
| Study title | Interventions | Study location | Status |
|---|
| Duvelisib to Combat COVID-19 | Drug: Duvelisib | United states | New |
| Inhaled Nitric Oxide for Preventing Progression in COVID-19 | Drug: Nitric Oxide | United states | Recruiting (New) |
| COVID-19 Convalescent Plasma (CCP) Transfusion | Biological: COVID Convalescent Plasma | United states | Recruiting (New) |
| VA Remote and Equitable Access to COVID-19 Healthcare Delivery (VA-REACH TRIAL) | Drug: HydroxychloroquineDrug: AzithromycinDrug: Placebo oral tablet | United states | Active, not recruiting. |
| Baricitinib, Placebo and Antiviral Therapy for the Treatment of Patients with Moderate and Severe COVID-19 | Drug: BaricitinibDrug: HydroxychloroquineDrug: Placebo administration | United states | Recruiting |
| Safety and Immunogenicity Study of 2019-nCoV Vaccine (mRNA-1273) for Prophylaxis of SARS-CoV-2 Infection (COVID-19) | Biological: mRNA-1273 | United states | Recruiting |
(Data is taken from US National Library of Medicine (https://www.nlm.nih.gov/))
Conclusion(s)
We still need to understand what, and how the virus have changed and effect the humans. Critical question is whether the sequencing will help to address this situation including an understanding of how effective the treatment strategies and interventions are, whether we are missing transmission chains, how locations of virus are connected to the transmission, and what are the factors that contribute to its spread. We are in the emerging stage of understanding that how the immunity mediates between both viral control and host toxicity during severe COVID-19. The knowledge gained from years of fundamental work in viral immunology will be needed for the test and treatment options that recruit both innate and adaptive immune systems for the prevention and treatment of COVID-19 and other future viral pandemics. An early identification of patients infected with SARS-CoV-2 is very important and beneficial to reduce the mortality in patients with severe symptoms associated with COVID-19. There is a need to expand the testing facilities for COVID-19, which will play an important role to find new and suspected cases as quickly as possible. Only then, we can isolate patients and break down the transmission.
[1]. [Internet]. Who.int. 2020 [cited 20 June 2020]. Available from: https://www.who.int/docs/default-source/coronaviruse/who-china-joint-mission-on-covid-19-final-report.pdf [Google Scholar]
[2]. Su S, Wong G, Shi W, Liu J, Lai A, Zhou J, Epidemiology, genetic recombination, and pathogenesis of coronavirusesTrends in Microbiology 2016 24(6):490-502.10.1016/j.tim.2016.03.00327012512 [Google Scholar] [CrossRef] [PubMed]
[3]. Cavanagh D, Coronavirus avian infectious bronchitis virusVeterinary Research 2007 38(2):281-97.10.1051/vetres:200605517296157 [Google Scholar] [CrossRef] [PubMed]
[4]. Ismail M, Tang Y, Saif Y, Pathogenicity of Turkey coronavirus in turkeys and chickensAvian Diseases 2003 47(3):515-22.10.1637/591714562877 [Google Scholar] [CrossRef] [PubMed]
[5]. Groneberg D, Hilgenfeld R, Zabel P, Molecular mechanisms of Severe Acute Respiratory Syndrome (SARS)Respiratory Research 2005 6(1)10.1186/1465-9921-6-815661082 [Google Scholar] [CrossRef] [PubMed]
[6]. Peiris JSM, Lui ST, Poon LLM, Guan Y, Yam LYC, Lim W, Coronavirus as a possible cause of severe acute respiratory syndromeThe Journal of Tepecik Education and Research Hospital 2003 13(1):55-56.10.5222/terh.2003.26734 [Google Scholar] [CrossRef]
[7]. Drosten C, Günther S, Preiser W, van der Werf S, Brodt H, Becker S, Identification of a novel coronavirus in patients with severe acute respiratory syndromeNew England Journal of Medicine 2003 348(20):1967-76.10.1056/NEJMoa03074712690091 [Google Scholar] [CrossRef] [PubMed]
[8]. Ksiazek T, Erdman D, Goldsmith C, Zaki S, Peret T, Emery S, A Novel coronavirus associated with severe acute respiratory syndromeNew England Journal of Medicine 2003 348(20):1953-66.10.1056/NEJMoa03078112690092 [Google Scholar] [CrossRef] [PubMed]
[9]. Reilley B, Van Herp M, Sermand D, Dentico N, SARS and Carlo UrbaniNew England Journal of Medicine 2003 348(20):1951-52.10.1056/NEJMp03008012748315 [Google Scholar] [CrossRef] [PubMed]
[10]. Zaki A, van Boheemen S, Bestebroer T, Osterhaus A, Fouchier R, Isolation of a novel coronavirus from a man with pneumonia in Saudi ArabiaNew England Journal of Medicine 2012 367(19):1814-20.10.1056/NEJMoa121172123075143 [Google Scholar] [CrossRef] [PubMed]
[11]. MoHFW | Home [Internet]. Mohfw.gov.in. 2020 [cited 20 June 2020]. Available from: https://www.mohfw.gov.in/ [Google Scholar]
[12]. COVID19 India Tracker Web App [Internet]. COVID19 India tracker. 2020 [cited 20 June 2020]. Available from: https://www.coronatracker.in/ [Google Scholar]
[13]. Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive studyThe Lancet 2020 395(10223):507-13.10.1016/S0140-6736(20)30211-7 [Google Scholar] [CrossRef]
[14]. Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, Clinical characteristics of 138 hospitalised patients with 2019 novel coronavirus-infected pneumonia in Wuhan, ChinaJAMA 2020 323(11):106110.1001/jama.2020.158532031570 [Google Scholar] [CrossRef] [PubMed]
[15]. The novel coronavirus outbreak: What we know and what we don’tCell 2020 180(6):1034-36.10.1016/j.cell.2020.02.02732078801 [Google Scholar] [CrossRef] [PubMed]
[16]. Li Q, Guan X, Wu P, Wang X, Zhou L, Tong Y, Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumoniaNew England Journal of Medicine 2020 382(13):1199-207.10.1056/NEJMoa200131631995857 [Google Scholar] [CrossRef] [PubMed]
[17]. Park W, Kwon N, Choi S, Kang C, Choe P, Kim J, Virus isolation from the first patient with SARS-CoV-2 in KoreaJournal of Korean Medical Science 2020 35(7)10.3346/jkms.2020.35.e8432080990 [Google Scholar] [CrossRef] [PubMed]
[18]. Zhang H, Zhou P, Wei Y, Yue H, Wang Y, Hu M, Histopathologic changes and SARS-CoV-2 immunostaining in the lung of a patient with COVID-19Annals of Internal Medicine 2020 172(9):629-32.10.7326/M20-053332163542 [Google Scholar] [CrossRef] [PubMed]
[19]. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Clinical features of patients infected with 2019 novel coronavirus in Wuhan, ChinaThe Lancet 2020 395(10223):497-506.10.1016/S0140-6736(20)30183-5 [Google Scholar] [CrossRef]
[20]. Zhang B, Zhou X, Qiu Y, Feng F, Feng J, Jia Y, Clinical characteristics of 82 death cases with COVID-19. Preprint at medRxiv 2020 10.1371/journal.pone.023545832645044 [Google Scholar] [CrossRef] [PubMed]
[21]. Special expert group for control of the epidemic of novel coronavirus pneumonia of the Chinese preventive medicine association. An update on the epidemiological characteristics of novel coronavirus pneumonia (COVID-19)Chin J Epidemiol 2020 41(2):139-44. [Google Scholar]
[22]. Guan W, Ni Z, Hu Y, Liang W, Ou C, He J, Clinical characteristics of coronavirus disease 2019 in ChinaNew England Journal of Medicine 2020 382(18):1708-20.10.1056/NEJMoa200203232109013 [Google Scholar] [CrossRef] [PubMed]
[23]. Wu Z, McGoogan J, Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) Outbreak in ChinaJAMA 2020 323(13):123910.1001/jama.2020.264832091533 [Google Scholar] [CrossRef] [PubMed]
[24]. Tang N, Bai H, Chen X, Gong J, Li D, Sun Z, Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathyJournal of Thrombosis and Haemostasis 2020 18(5):1094-99.10.1111/jth.1481732220112 [Google Scholar] [CrossRef] [PubMed]
[25]. Guo T, Fan Y, Chen M, Wu X, Zhang L, He T, Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19)JAMA Cardiology 2020 10.1001/jamacardio.2020.101732219356 [Google Scholar] [CrossRef] [PubMed]
[26]. Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort studyThe Lancet 2020 395(10229):1054-62.10.1016/S0140-6736(20)30566-3 [Google Scholar] [CrossRef]
[27]. Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H, Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: A single-centered, retrospective, observational studyThe Lancet Respiratory Medicine 2020 8(5):475-81.10.1016/S2213-2600(20)30079-5 [Google Scholar] [CrossRef]
[28]. Qin C, Zhou L, Hu Z, Zhang S, Yang S, Tao Y, Dysregulation of immune response in patients with COVID-19 in Wuhan, ChinaSSRN Electronic Journal 2020 10.2139/ssrn.3541136 [Google Scholar] [CrossRef]
[29]. Zhou L, Liu K, Liu HG, Cause analysis and treatment strategies of “recurrence” with novel coronavirus pneumonia (COVID-19) patients after discharge from hospitalJournal of Tuberculosis Respiratory Disease 2020 43(4):281-84. [Google Scholar]
[30]. Wu F, Zhao S, Yu B, Chen Y, Wang W, Song Z, A new coronavirus associated with human respiratory disease in ChinaNature 2020 579(7798):265-69.10.1038/s41586-020-2008-332015508 [Google Scholar] [CrossRef] [PubMed]
[31]. Catanzaro M, Fagiani F, Racchi M, Corsini E, Govoni S, Lanni C, Immune response in COVID-19: Addressing a pharmacological challenge by targeting pathways triggered by SARS-CoV-2Signal Transduction and Targeted Therapy 2020 5(1)10.1038/s41392-020-0191-132467561 [Google Scholar] [CrossRef] [PubMed]
[32]. Magrone T, Magrone M, Jirillo E, Focus on receptors for coronaviruses with special reference to angiotensin-converting enzyme 2 as a potential drug target- A perspectiveEndocr Metab Immune Disord Drug Targets 2020 Apr 27 10.2174/187153032066620042711290232338224 [Google Scholar] [CrossRef] [PubMed]
[33]. Du S, Yuan W, Mathematical modeling of interaction between innate and adaptive immune responses in COVID-19 and implications for viral pathogenesisJournal of Medical Virology 2020 10.1002/jmv.2586632356908 [Google Scholar] [CrossRef] [PubMed]
[34]. Tay M, Poh C, Rénia L, MacAry P, Ng L, The trinity of COVID-19: immunity, inflammation and interventionNature Reviews Immunology 2020 20(6):363-74.10.1038/s41577-020-0311-832346093 [Google Scholar] [CrossRef] [PubMed]
[35]. Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, Pathological findings of COVID-19 associated with acute respiratory distress syndromeThe Lancet Respiratory Medicine 2020 8(4):420-22.10.1016/S2213-2600(20)30076-X [Google Scholar] [CrossRef]
[36]. Choi IA, Lee SJ, Park W, Park SH, Shim SC, Baek HJ, Effects of Tocilizumab therapy on serum interleukin-33 and interleukin-6 levels in patients with rheumatoid arthritisArchives of Rheumatology 2018 33(4):389-94.10.5606/ArchRheumatol.2018.675330874247 [Google Scholar] [CrossRef] [PubMed]
[37]. Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, A pneumonia outbreak associated with a new coronavirus of probable bat originNature 2020 579(7798):270-73.10.1038/s41586-020-2012-73201550 [Google Scholar] [CrossRef] [PubMed]
[38]. Xu X, Chen P, Wang J, Feng J, Zhou H, Li X, Evolution of the novel coronavirus from the ongoing Wuhan outbreak and modeling of its spike protein for risk of human transmissionScience China Life Sciences 2020 63(3):457-60.10.1007/s11427-020-1637-532009228 [Google Scholar] [CrossRef] [PubMed]
[39]. Wrapp D, Wang N, Corbett KS, Goldsmith JA, Hsieh CL, Abiona O, Cryo-EM structure of the 2019-nCoV spike in the prefusion conformationScience 2020 367(6483):1260-63.10.1126/science.abb250732075877 [Google Scholar] [CrossRef] [PubMed]
[40]. Zhu H, Shi X, Ju D, Huang H, Wei W, Dong X, Anti-inflammatory effect of thalidomide on H1N1 influenza virus-induced pulmonary injury in miceInflammation 2014 37:2091-98.10.1007/s10753-014-9943-924912813 [Google Scholar] [CrossRef] [PubMed]
[41]. Keddie S, Bharambe V, Jayakumar A, Shah A, Sanchez V, Adams A, Clinical perspectives into the use of thalidomide for central nervous system tuberculosisEur J Neurol 2018 25:1345-51.10.1111/ene.1373229935038 [Google Scholar] [CrossRef] [PubMed]
[42]. Haraf R, Flora AS, Assaly R, Thalidomide as a cough suppressant inidiopathic pulmonary fibrosisAmerican Journal of Therapeutics 2018 25(6):e687-88.10.1097/MJT.000000000000069529232284 [Google Scholar] [CrossRef] [PubMed]
[43]. Chen C, Qi F, Shi K, Li Y, Li J, Chen Y, Thalidomide combined with low-dose glucocorticoid in the treatment of COVID-19 pneumoniaPreprints 2020 :2020020395published online February 26. DOI:10.1101/202002.0395.v1(preprint) [Google Scholar]
[44]. Tanaka T, Narazaki M, Kishimoto T, Immunotherapeutic implications of IL-6 blockade for cytokine stormImmunotherapy 2016 8:959-70.10.2217/imt-2016-002027381687 [Google Scholar] [CrossRef] [PubMed]
[45]. Shakoory B, Carcillo JA, Chatham WW, Amdur RL, Zhao H, Dinarello CA, Interleukin-1 receptor blockade is associated with reduced mortality in sepsis patients with features of macrophage activation syndrome: reanalysis of a prior phase III trialCritical Care Medicine 2016 44:275-81.10.1097/CCM.000000000000140226584195 [Google Scholar] [CrossRef] [PubMed]
[46]. Broglie L, Pommert L, Rao S, Thakar M, Phelan R, Margolis D, Ruxolitinib for treatment of refractory hemophagocytic lymphohistiocytosisBlood Advances 2017 1(19):1533-36.10.1182/bloodadvances.201700752629296794 [Google Scholar] [CrossRef] [PubMed]
[47]. Sin JH, Zangardi ML, Ruxolitinib for secondary hemophagocytic lymphohistiocytosis: First case reportHematol Oncol Stem Cell Ther 2019 12:166-70.10.1016/j.hemonc.2017.07.00228834694 [Google Scholar] [CrossRef] [PubMed]
[48]. Ahmed A, Merrill SA, Alsawah F, Bockenstedt P, Campagnaro E, Devata S, Ruxolitinib in adult patients with secondary haemophagocytic lymphohistiocytosis: An open label, single-centre, pilot trialLancet Haematology 2019 6:e630-37.10.1016/S2352-3026(19)30156-5 [Google Scholar] [CrossRef]
[49]. Chan MCW, Kuok DIT, Leung CYH, Hui KPY, Valkenburg SA, Lau EHY, Human mesenchymal stromal cells reduce influenza A H5N1-associated acute lung injury in vitro and in vivoProc Natl Acad Sci USA 2016 113:3621-26.10.1073/pnas.160191111326976597 [Google Scholar] [CrossRef] [PubMed]
[50]. Yang BX, El Farran CA, Guo HC, Yu T, Fang HT, Wang HF, Systematic identification of factors for provirus silencing in embryonic stem cellsCell 2015 163:230-45.10.1016/j.cell.2015.08.03726365490 [Google Scholar] [CrossRef] [PubMed]
[51]. Simonson OE, Mougiakakos D, Heldring N, Bassi G, Johansson HJ, Dalén M, In vivo effects of mesenchymal stromal cells in two patients with severe acute respiratory distress syndromeStem Cells Transl Med 2015 4:1199-213.10.5966/sctm.2015-002126285659 [Google Scholar] [CrossRef] [PubMed]
[52]. Zheng G, Huang L, Tong H, Shu Q, Hu Y, Ge M, Treatment of acute respiratory distress syndrome with allogeneic adipose-derived mesenchymal stem cells: A randomized, placebo-controlled pilot studyRespir Res 2014 15:3910.1186/1465-9921-15-3924708472 [Google Scholar] [CrossRef] [PubMed]
[53]. Winkler AM, Koepsell SA, The use of convalescent plasma to treat emerging infectious diseases: Focus on Ebola virus diseaseCurr Opin Hematol 2015 22:521-26.10.1097/MOH.000000000000019126457963 [Google Scholar] [CrossRef] [PubMed]
[54]. Li H, Zhou Y, Zhang M, Wang H, Zhao Q, Liu J, Updated approaches against SARS-CoV-2Antimicrob Agents Chemother 2020 64(6):e00483-20.10.1128/AAC.00483-20 [Google Scholar] [CrossRef]
[55]. Blaising J, Polyak SJ, Pécheur EI, Arbidol as a broad-spectrum antiviral: An updateAntiviral Res 2014 107:84-94.10.1016/j.antiviral.2014.04.00624769245 [Google Scholar] [CrossRef] [PubMed]
[56]. Wang M, Cao R, Zhang L, Yang X, Liu J, Xu M, Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitroCell Res 2020 30(3):269-71.10.1038/s41422-020-0282-032020029 [Google Scholar] [CrossRef] [PubMed]
[57]. Gao J, Tian Z, Yang X, Breakthrough: Chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studiesBiosci Trends 2020 14(1):72-73.10.5582/bst.2020.0104732074550 [Google Scholar] [CrossRef] [PubMed]