According to Global Cancer Statistics (GLOBOCAN) 2018, Worldwide, it is estimated that about 2.1 million are newly diagnosed female breast cancer cases in 2018, accounting for almost 1 in 4 cancer cases among women [1]. The incidence of breast cancer is higher in developed countries, while relative mortality is more in less developed countries [2]. Carcinoma breast is now the most common cancer in Indian women having recently surpassed cervical cancer [3]. Among Indian females age adjusted breast cancer rate is as high as 25.8 per 100,000 women and mortality is 12.7 per 100,000 women [4]. Clinical examination of breast has its limitations in evaluation of dense breast, deep seated lesions and in detecting early lesions in asymptomatic women. Various imaging modalities like Mammography, Contrast enhanced digital mammography, Ultrasound breast, Breast tomosynthesis, MRI breast and Molecular Imaging of breast are used for screening and evaluation of breast lesions [5].
Mammography is a commonly used imaging modality which is especially useful in screening of breast cancers. The utility of mammography is limited if the breast parenchyma is dense. Although very small radiation dose is administered in mammography [5], the risk of radiation induced cancer is still present. Breast ultrasound is a useful imaging modality in assessing dense breasts, palpable lump, detecting and characterising lesions in pregnant and lactating women. Ultrasound lacks radiation, can complement mammography, but has certain limitations like operator dependency [6,7].
In Digital Breast Tomosynthesis (DBT), the breast is viewed in a three-Dimensional (3D) format (also called 3D Mammography) as multiple thin-slice images spanning the entire breast somewhat similar to a Computed Tomography (CT) scan. It has advantages over routine mammography in detection and characterisation of breast lesions [8].
MRI has an excellent soft tissue resolution and can detect lesions which are not seen on mammography or ultrasonography [9]. MRI breast is a very useful modality for preoperative staging of breast cancer, for evaluation of extent of disease in ipsilateral/contralateral breasts and chest wall in newly diagnosed breast cancer, for evaluation of unilateral metastatic axillary lymphadenopathy with unknown primary, for screening women at high risk for breast cancer, to know response to neoadjuvant chemotherapy and evaluation of breast implants [10]. DCE MRI is better for characterisation of fibroglandular tissue and breast lesions as it improves specificity of MRI. Kinetic Curves (KC) are derived from DCE MRI according to the wash in and wash out patterns of intravenous contrast [11].
Lee RK et al., did a retrospective study on 29 patients with Lobular Carcinoma In Situ (LCIS of Breast) who underwent initial excision. After initial excision, breast ultrasonography and breast MRI were carried out and imaging findings were compared with pathologic results. It was concluded that breast MRI has higher sensitivity than breast ultrasound in detecting remnant LCIS lesions [12]. Giess CS et al., did a retrospective review study using MRI database of 7332 contrast enhanced breast MRI examinations of 294 women for inconclusive mammographic findings. The authors concluded that MRI is a problem solving tool for inconclusive mammographic findings [13]. Li E et al., conducted a study including 91 patients with micro calcifications of BI-RADS grade 3-5 on mammography to investigate the diagnostic value of breast MRI. The authors concluded that breast MRI has the potential to improve the diagnosis of BI-RADS category IV micro calcifications on mammography with better specificity, PPV and accuracy [14]. Razek NMA et al., did a retrospective review study on 56 cases of Invasive Lobular breast Carcinoma (ILC) in which mammography, ultrasonography and dynamic MRI were considered. The pathology records of all cases were available for review. It was concluded that MRI is superior to mammography and ultrasonography in the detection and management of ILC [15].
Although there are many studies on Breast MRI, there are very few studies in tertiary care centres especially in Northern India [16,17]. Hence, the present study was carried out with an aim to evaluate the efficacy of DCE MRI in detection and characterisation of breast lesions according to KC analysis and to correlate MRI findings with pathological findings.
Materials and Methods
This prospective observational study was conducted at Guru Gobind Singh Medical College and Hospital, Faridkot, Punjab, India, from February 2019 to December 2019 after obtaining Institutional Ethical Committee (IEC) clearance. DCE MRI was performed after informed consent in 50 females (Age ranging from 17 years to 80 years). A non-random convenient sampling technique was adopted on consecutively eligible participants referred for MRI presenting with a clinical diagnosis of breast lesion.
Sample Size Calculation
The sample size was calculated using following formulae:
n=(Zα/2)2 * (PQ)/E2
n-Sample size
Zα/2-Z value at 5% error (1.96)
P-Taken as 37% [18]
Q=1-P
E-Absolute error (taken as 20%)
Sample size was found to be 23 by using this formula. However, 50 consecutive eligible participants were taken in the present study. The present study included clinically symptomatic participants presenting to the Radiodiagnosis Department for MRI breast with complaints of breast lump, pain, discharge. Any participant having contraindication for MRI (patients with metallic implants, patients with claustrophobia, contraindications to gadolinium-based contrast media due to allergy, pregnancy, or compromised renal function (eGFR <30 mL/min/m2) were excluded from the study. Participants who were not able to lie to prone or were having marked kyphosis or kyphoscoliosis, marked obesity, extremely large breasts; implantable devices that are not MRI compatible were also excluded from the study.
MRI was performed on Siemens “MAGNETOM Avanto” 1.5 Tesla using a standard dedicated breast matrix coils. The standard MRI protocol included the following pulse sequences:
Axial two-Dimentional (2D) T2-Weighted short tau inversion recovery turbo spin-echo pulse sequence
Pre-contrast and post-contrast enhanced fat-saturated axial 3D T1-Weighted fast low-angle shot volume-interpolated breath-hold examination pulse sequences.
Axial 3D delayed contrast-enhanced turbo spin-echo pulse sequence was performed for the evaluation of the supraclavicular and axillary lymph nodes. Six dynamic sequences were performed before and after intravenous contrast injection. The contrast medium Meglumine-Gadoterate (0.1 mmol/kg body weight) was injected intravenously followed by a 20-mL saline flush. Post processing manipulation included standard subtraction, reverse subtraction, MIP images and KCs.
The BI-RADS classification [Table/Fig-1] proposed by the American College of Radiology (ACR), 5th edition in 2013 was used in characterising lesions in the present study. Use of Category “0” should be avoided and Sub Categories 4A, 4B and 4C are not used for breast MRI reporting [19].
Breast Imaging-Reporting and Data System (BI-RADS) LEXICON [19].
| Category | Likelihood of cancer | Imaging findings |
|---|
| 0 | N/A | Incomplete, further imaging or information is required |
| I | Essentially 0% | Negative, symmetrical and no masses, architectural disturbances or suspicious calcifications present |
| II | Essentially 0% | Benign findings |
| III | >0% but ≤2% | Probably benign, short interval follow-up suggested |
| IV | 4a. Low suspicion for malignancy (>2% to ≤10%) | Imaging Findings (Suspicious Abnormality) |
| 4b. Moderate suspicion for malignancy (>10% to ≤50%) |
| 4c. High suspicion for malignancy (>50% to <95%) |
| V | >95% | Highly suggestive of malignancy, action should be taken |
| VI | N/A | Known biopsy proven malignancy |
Breast MRI interpretation and reporting should include documentation of Background Parenchymal Enhancement (BPE) along with enhancement of patient’s fibroglandular tissue (ranging from almost entirely fatty to extreme fibroglandular characteristics) [19]. BPE is assessed at approximately 90 seconds in the postcontrast images and can be minimal, mild, moderate or marked-probability of breast cancer increases with higher BPE. There are predominantly three terminologies used in Breast MRI which include focus, a mass, or an area of Non Mass Enhancement (NME). A focus by definition is a unique punctate enhancing dot, usually <5 mm, lacking the characteristics of a mass. If a lesion can be described in 3-D and has convex outer margins-it is by definition a mass. NME is defined as enhancement which stands out compared to BPE but by definition is neither a mass nor a focus. It is recommended to use fat saturation to maximise the contrast between enhancing cancers and breast fat, which is bright on MRI. Subtraction is also done to accentuate the enhancing abnormalities on the study [20].
The differences due to increased vascular permeability of cancers as compared to normal breast tissue have been utilised in DCE MRI which takes into consideration the enhancement characteristics of a lesion over time. ACR have described three patterns of KCs in BI-RADS Atlas. Early enhancement which can be (slow, medium, and fast) is usually assessed upto 90 to 120 seconds and the delayed enhancement after the peak (persistent, plateau, and washout) is assessed with endpoint at 4-5 minutes [20-22]. Cancers typically show fast initial and delayed washout KC. The kinetic characteristics of the Region of Interest (ROI) may be assessed either visually or computer-aided detection which can also provide colour-coded images. The morphological and kinetic characteristics of a lesion are assessed along with associated findings like skin thickening/nipple retraction or chest wall invasion, altered signal intensity in ducts, lymphadenopathy [23].
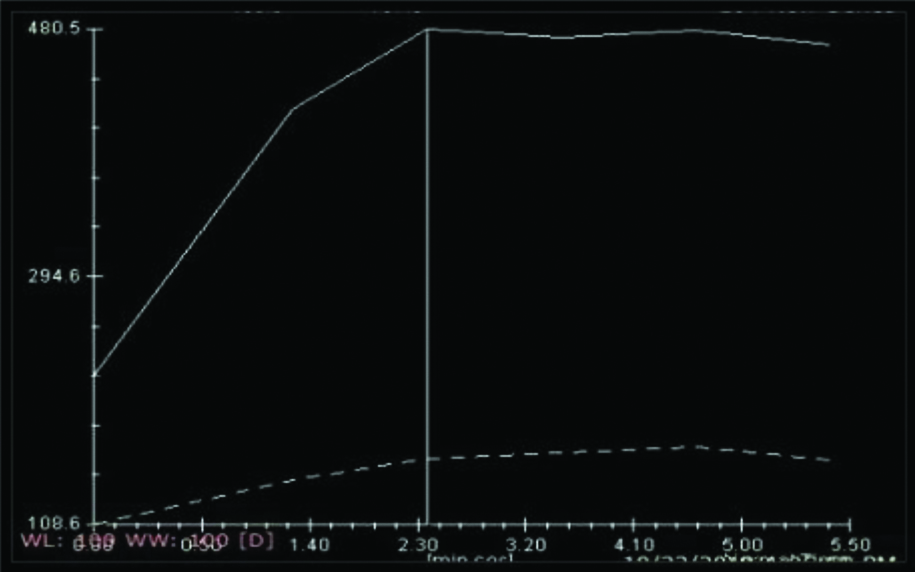
Type I curve [Table/Fig-2] shows progressive, continuous enhancement pattern which is usually associated with benign conditions [24,25].
Showing Kinetic Curve (KC) analysis Type 1 curve.
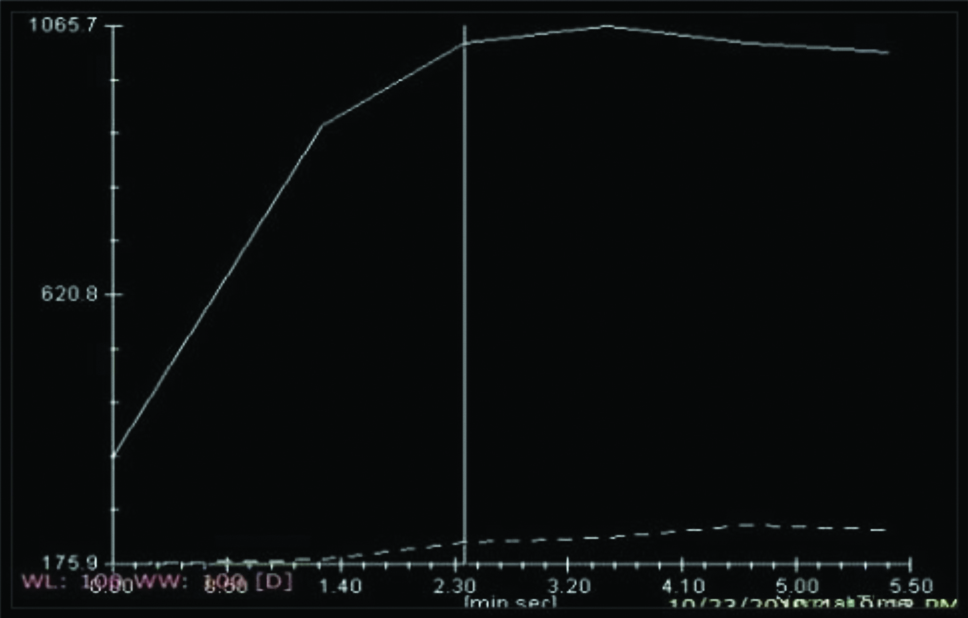
Type II curve [Table/Fig-3] is a plateau pattern, in which an initial increase in signal intensity is followed by a plateau phase and has a higher specificity for the detection of malignancy [26].
Showing Kinetic Curve (KC) analysis Type 2 curve.
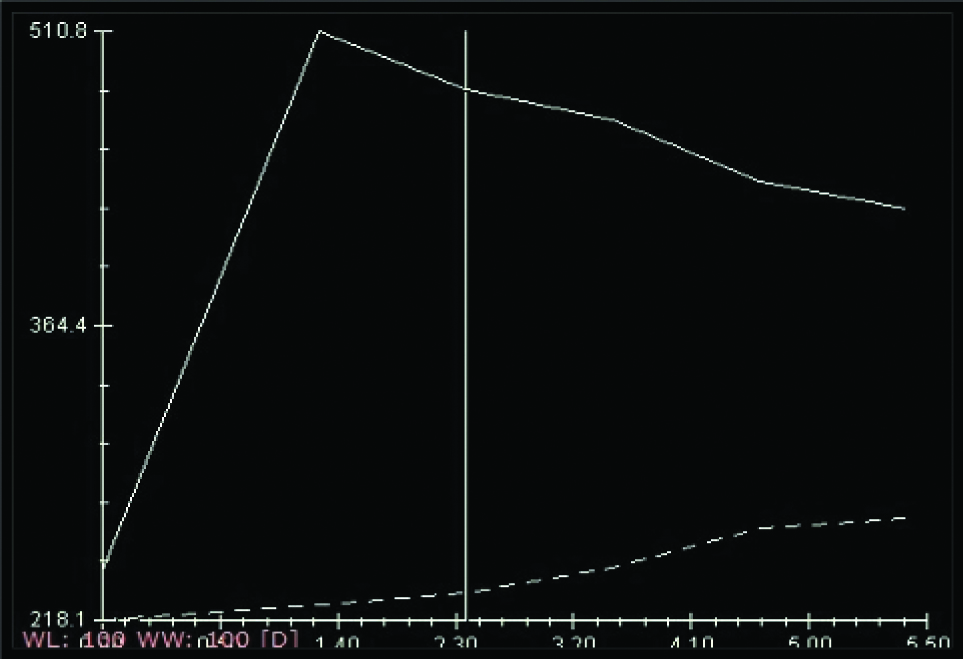
Type III curve [Table/Fig-4] a washout enhancement pattern, involves an initial increase followed by decreased enhancement in delayed phase. Should be considered suggestive of malignancy and is not usually seen in patients with benign lesions [26].
Showing Kinetic Curve (KC) analysis Type 3 curve.
Morphological Criteria for Malignancy
The shape, margin and internal enhancement characteristics have to be analysed to differentiate it as benign or malignant. Irregular shape, non-circumscribed margin and rim like enhancement pattern favours malignancy. Irregular or spiculated margins [Table/Fig-5] with heterogeneous internal enhancement [Table/Fig-6] raise the concern for malignancy [27-29]. Segmental or clumped enhancement is more likely to indicate malignancy in a NME [28].
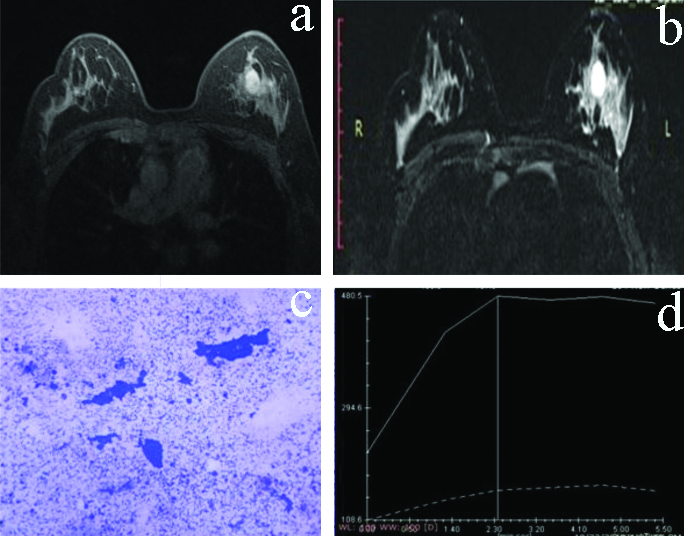
a,b) T2W and T1W post contrast axial images-An irregular lesion in right breast appearing hyperintense on T2W image with in homogeneous postcontrast enhancement on T1W postcontrast image. c) HPE 40x. Features s/o Ca breast. d) Dynamic KC analysis: type II curve.
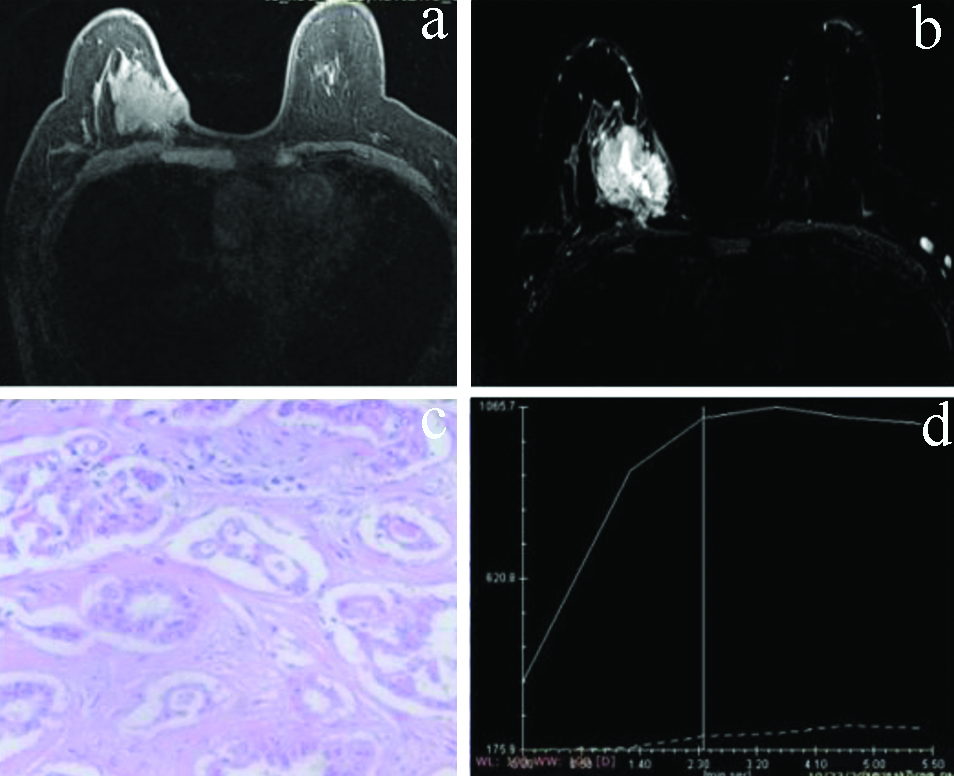
T2W and T1W postcontrast axial images: T2W and T1W post contrast axial images-A spiculated lesion in right breast appearing hyperintense on T2W image with heterogeneous postcontrast enhancement on T1W postcontrast image. c) 40x Giemsa stained smear. Features s/o Ca breast. d) Dynamic KC analysis: showing type III curve.
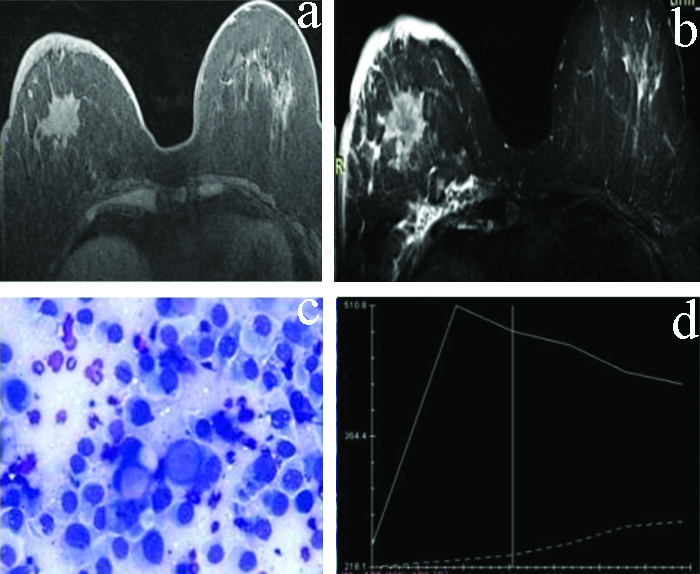
T2-weighted Signal Characteristics
T2-weighted signal hyperintensity within the viable (enhancing) portion of the lesion [Table/Fig-7] strongly favours benign aetiology [30]. On T2-weighted images, breast cancers usually have similar or decreased signal intensity, compared to the normal breast parenchyma [31]. Peri-lesional oedema, architectural distortion, skin thickening and lymphadenopathy favour malignancy [30,32].
a,b) T2W and T1W postcontrast axial images: a T2 hyper intense well circumscribed oval lesion showing homogenous enhancement. c) HPE 40x. Features s/o Ca breast. d) Dynamic KC analysis: showing type 1 curve.
Statistical Analysis
The data thus obtained was statistically analysed by using SPSS version 20 (Chicago, Inc, USA). Sensitivity, specificity, PPV, NPV of MRI were calculated and compared with pathological findings. Chi-square test was used to compare categorical data. A p value <0.05 was considered statistically significant.
Results
The cases were taken from 17 years to 80 years of age group. The mean age of cases was 51.42±16.12 years [Table/Fig-8]. The most common presenting symptoms was lump in both benign and malignant lesions (57 lesions, 89.1%) [Table/Fig-9]. Out of 50 patients, 08 (16%) showed multifocal/multicentric lesions, 03 cases (6%) had bilateral breast lesions [Table/Fig-10]. BPE was assessed and the maximum number of patients (34 out of 50) showed minimal BPE [Table/Fig-11]. Maximum number of lesions was of BI-RADS category V [Table/Fig-12].
Distribution of cases according to age.
| Age (Years) | Frequency | Percent |
|---|
| 17-26 | 8 | 16.0 |
| 27-36 | 8 | 16.0 |
| 37-46 | 7 | 14.0 |
| 47-56 | 5 | 10.0 |
| 57-66 | 16 | 32.0 |
| 67-76 | 5 | 10.0 |
| >76 | 1 | 2.0 |
| Total | 50 | 100.0 |
| Mean±SD | 51.42±16.12 |
Distribution of frequency of lesions according to complaints.
| Complaint | Frequency | Percent |
|---|
| Lump | 57 | 89.1 |
| Discharge | 3 | 4.6 |
| Pain | 2 | 3.1 |
| Discharge+Lump | 1 | 1.6 |
| Lump+Pain | 1 | 1.6 |
| Total | 64 | 100.0 |
Distribution of cases according to origin.
| Number of cases | Percentage |
|---|
| Unilateral/Focal | 39 | 78 |
| Multifocal/Multicenteric | 8 | 16 |
| Bilateral | 3 | 6 |
| Total | 50 | 100 |
Distribution of cases according to BPE.
| Background Parenchymal Enhancement (BPE) | Number of cases | Percentage |
|---|
| Minimal | 34 | 68.0 |
| Mild | 15 | 30.0 |
| Moderate | 1 | 2.0 |
| Marked | 0 | 0.0 |
| Total | 50 | 100.0 |
Distribution of lesions according to BI-RADS category.
| BI-RADS category (MRI) | Number of lesions |
|---|
| BI-RADS-I | 4 |
| BI-RADS-II | 4 |
| BI-RADS-III | 19 |
| BI-RADS-IV | 7 |
| BI-RADS-V | 30 |
| Total | 64 |
Both morphologic characteristics and KC analysis of the lesions was included in the study. According to which 20 lesions showed type I curve, 14 lesions showed type II curve and 23 lesions showed type III curve and 7 lesions did not show any enhancement [Table/Fig-13]. Out of the enrolled 64 lesions in present study, 24 lesions were benign, 36 were malignant and 4 clinically symptomatic cases with pain were turned out to be normal after both morphological and KC analysis on MRI [Table/Fig-14]. On pathology out of 64 lesions, 25 lesions were benign, 34 were malignant and 5 turned out to be normal [Table/Fig-15].
Distribution of lesions according to Kinetic Curve (KC) analysis on MRI.
| KC type | MRI | Total |
|---|
| Benign | Malignant | Normal |
|---|
| N/A | 3 | 0 | 4 | 7 |
| 42.9% | 0% | 57.1% | 100% |
| 1 | 16 | 4 | 0 | 20 |
| 80.0% | 20.0% | 0% | 100% |
| 2 | 5 | 9 | 0 | 14 |
| 35.7% | 64.3% | 0% | 100.0% |
| 3 | 0 | 23 | 0 | 23 |
| 0% | 100% | 0% | 100% |
| Total | 24 | 36 | 4 | 64 |
| 37.5% | 56.2% | 6.2% | 100% |
| p-value | 0.001 (Sig.) |
Test applied: chi-square test
N/A: Not available
Distribution of lesions in different age groups according to MRI findings.
| Age (Years) | MRI | Total |
|---|
| Benign | Malignant | Normal |
|---|
| 17-26 | 7 | 1 | 2 | 10 |
| 10.9% | 1.6% | 3.1% | 15.6% |
| 27-36 | 6 | 0 | 2 | 8 |
| 9.4% | 0% | 3.1% | 12.5% |
| 37-46 | 6 | 6 | 0 | 12 |
| 9.4% | 9.4% | 0% | 18.8% |
| 47-56 | 0 | 5 | 0 | 5 |
| 0% | 7.8% | 0% | 7.8% |
| 57-66 | 5 | 18 | 0 | 23 |
| 7.8% | 28.1% | 0% | 35.9% |
| 67-76 | 0 | 5 | 0 | 5 |
| 0% | 7.8% | 0% | 7.8% |
| >76 | 0 | 1 | 0 | 1 |
| 0% | 1.6% | 0% | 1.6% |
| Total | 24 | 36 | 4 | 64 |
| 37.5% | 56.3% | 6.2% | 100.0% |
| p-value | 0.001 (Sig.) |
Test applied: chi-square test
Distribution of lesions according to pathological findings in different age groups.
| Age (Years) | Pathology | Total |
|---|
| Benign | Malignant | Normal |
|---|
| 17-26 | 8 | 0 | 2 | 10 |
| 12.5% | 0% | 3.1% | 15.6% |
| 27-36 | 5 | 0 | 3 | 8 |
| 7.8% | 0% | 4.7% | 12.5% |
| 37-46 | 8 | 4 | 0 | 12 |
| 12.5% | 6.3% | 0% | 18.8% |
| 47-56 | 0 | 5 | 0 | 5 |
| 0% | 7.8% | 0% | 7.8% |
| 57-66 | 4 | 19 | 0 | 23 |
| 6.3% | 29.7% | 0% | 35.9% |
| 67-76 | 0 | 5 | 0 | 5 |
| 0% | 7.8% | 0% | 7.8% |
| >76 | 0 | 1 | 0 | 1 |
| 0% | 1.6% | 0% | 1.6% |
| Total | 25 | 34 | 5 | 64 |
| 39.1% | 53.1% | 7.8% | 100.0% |
| p-value | 0.001 (Sig.) |
Test applied: chi-square test
The overall sensitivity and specificity of MRI were 98.18% and 55.56%, respectively whereas PPV and NPV of MRI were 93.10% and 83.33%, respectively [Table/Fig-16].
Sensitivity, specificity, Positive and Negative Predictive Values (PPV, NPV) of MRI Breast.
| Statistic | Value | 95% CI |
|---|
| Sensitivity | 98.18% | 90.28% to 99.95% |
| Specificity | 55.56% | 21.20% to 86.30% |
| Positive predictive value | 93.10% | 86.66% to 96.56% |
| Negative predictive value | 83.33% | 39.68% to 97.44% |
Discussion
In the present study, participating subjects were ranging from the age of 17 years to 80 years. Breast lump was the most common presenting complaint. Benign lesions were mostly seen in early age group (mean age 33.75 years). There was no benign lesion after the age of 66 years. With advancing age malignant lesions were commonly seen (mean age 63.2 years). There was only one suspicious lesion on MRI in below 17-26 years age group which was benign on pathology. In the present study, 16% cases showed multifocal/multicentric lesions and 6% had bilateral breast lesions. Similar findings were also seen in a meta-analysis done by Houssami N et al., in which multifocal or multicentric disease ranged from 6% to 34% [33]. Out of 25 benign lesions reported on pathological diagnosis about 5 (20%) showed associated axillary lymphadenopathy on MRI. Out of 36 malignant lesions reported on pathological diagnosis about 16 (44.44%) showed associated axillary lymphadenopathy on MRI. Out of 36 malignant lesions, 14 (21.87%) showed associated skin thickening.
In the present study, BPE was assessed which in 68% subjects was minimal, whereas in 30% it was mild and in remaining 2% moderate. None of the cases show marked BPE, in moderate/marked BPE a lesion may be obscured. Findings of the present study were similar to a study done by Sippo DA et al., in which 4686 screening MRI examinations were performed in 2446 women, BPE was reported as minimal or mild in 3975 (85%) examinations and moderate or marked in 711(15%) examinations [34]. The lesions were categorised according to the BI-RADS. For the purpose of correlation with pathology, BI-RADS I, II and III categories were taken as benign (Risk of malignancy <2%), while BI-RADS IV and V categories were taken as malignant. Out of 25 benign lesions (confirmed by pathology), 3 lesions were of BI-RADS II, 19 lesions were BI-RADS III and 3 lesions were of BI-RADS IV on MRI. Out of 34 malignant lesions (confirmed by pathology), 30 lesions were of BI-RADS V category and 4 cases were of BI-RADS IV category on MRI.
Out of 64 lesions, 6 lesions (6.25%) showed NME of which 2 (33.3%) were normal and 4 (66.6%) were benign on cytopathology. NME can represent both benign and malignant lesions. A study done by Ballesio L et al., in which out of 94 cases of NME, 73 cases (77.65%) showed malignant disease and 21 cases (22.34%) showed benign disease [35].
Out of 50 cases, 2 cases showed focus. Both these cases were not high risk patients so, were advised follow-up after six months but these cases didn’t turn up for follow-up. A Focus can represent benign or malignant pathology but usually benign pathology so, follow-up is required. A study done by Clauser P et al., taking 166 women (mean age 43 years), who underwent a median of four MRI examinations (range 2-6) during the study period. The results showed 68 foci in 58 women. The Authors concluded that foci are relatively frequent in screening MRI, and they are usually benign. An increase in size is the most reliable criteria to suspect malignancy [36]. In the present study on contrast enhanced MRI, 7 (10.9%) lesions showed no enhancement, of which 3 (42.9%) were benign and 4 (57.1%) were normal on cytopathology although the patients were clinically symptomatic. Type 1 KC was seen in 20 (31.3%) lesions. Out of them 15 (75%) were benign and 4 (20%) were malignant and 1 (5%) was normal on histopathology. Type 2 KC was seen in 14 (21.9%) lesions. Out of them 6 were benign (42.9%) and 8 were malignant (57.14%) on cytopathology. Type 3 KC was seen in 23 (35.9%) lesions. Out of them 1 (4.3%) was benign on cytopathology and 22 (95.7%) were malignant on pathology (20 on cytopathology and 2 on histopathology). Most of the malignant lesions showed washout kinetics (30 of 34, 96%). Most of benign lesions showed persistent kinetics (16 of 23, 69.56%). Plateau kinetics was seen in both benign and malignant cases (Benign in 6 out of 14, 42.85% and malignant in 8 out of 14, 57.14%). Study done by Jabbar SB et al., showed that most MRI lesions with persistent kinetics were benign (84%, 62 out of 74). Lesions with plateau kinetics showed intermediate results with majority being benign in nature (70%, 31 out of 44). About 30 lesions showed washout kinetics with 57% being benign (17 of 30) and 30% were malignant (9 of 30) [37]. Present study was in discordance with these results likely due to geographic distribution, high prevalence of cancer in population of Malwa region in Punjab from which we had taken the study group.
In the present study sensitivity of MRI to detect breast lesions was 98.18% and specificity, PPV and NPV were 55.56%, 93.10% and 83.33%, respectively. These results coincide with the study done by Fischer U et al., in which sensitivity and specificity of MRI were 93% and 65%, respectively [38]. There is a considerable overlap in the enhancement characteristics of benign and malignant lesions, therefore lesion characterisation based on kinetics assessment alone is not recommended [20,22,24]. The high PPV in the present study may be due to more number of positive cases and relatively less number of false positive cases in the present study. The low NPV in the present study may be due the lesser number of true negative patients in the study group or due to smaller study group.
Limitation(s)
The high prevalence of cancer in population of Malwa region of Punjab from where study subjects were considered may led to higher number of malignant lesions than benign lesions. The trend of using MRI as a problem solving modality in breast imaging after other imaging modalities may be another reason for higher PPV and lower NPV of MRI in present study.
Conclusion(s)
Magnetic Resonance Imaging (MRI) is a highly sensitive modality to detect breast lesions with a very high sensitivity of 98.18% and high PPV of 93.10%. The NPV of MRI is 83.33% and specificity is relatively low (55.56%). Both morphological characteristics and KC analysis should be used in combination for MRI breast interpretation to improve the specificity of MRI. Only KC analysis should not be used to differentiate benign from malignant lesions because of considerable overlap in enhancement characteristics between benign and malignant lesions.
Test applied: chi-square test
N/A: Not available
Test applied: chi-square test
Test applied: chi-square test