HIV and Acquired Immune Deficiency Syndrome (AIDS) have emerged as one of the most widespread public health problems throughout the world. The prevalence of HIV in adult population in India was estimated as 0.22% (0.16% – 0.30%) in the age group of 15-49 years with the prevalence of 0.25% (0.18-0.34) among males and 0.19% (0.14-0.25) among females as stated in the recently released India’s National HIV Report 2017 [1]. HIV not only affects the immunity of the patient by infecting the T-helper cells, but it also affects the microbiota of the patient [2,3]. In advance stages of infection, and full-blown AIDS, immunodeficiency stemming from failing host immune response and immune-surveillance due to falling CD4 T cells renders the host weak enough to acquire diverse opportunistic infections, neoplasms and develop many other unfavourable physiological alterations practically affecting every system [3,4]. The use of HAART has extended the life expectancy of HIV-infected individuals. However, opportunistic infections continue to remain a serious problem even in patients undergoing ART [5]. STI are known to increase the risk of contracting or transmitting HIV infection. The risk-enhancement correlations of STIs and HIV infection are reciprocal [6,7]. HIV seropositive women are at a high risk of acquiring VVIs, which have been reported to be as high as 72% compared to 6% reported in their HIV seronegative counterparts [8]. The dynamics of vaginitis among HIV/AIDS patients are greatly influenced by the pathogens involved, thus treating vaginitis, (even if asymptomatic), assumes greater significance among HIV/AIDS patients [9].
To prevent recurrences of VVI by multi-drug resistant pathogens and to avoid overwhelming infection, it is important to identify the organism and outline specific preventive strategy even in asymptomatic HIV seropositive women. The current challenge is to develop rapid and specific detection of each species of pathogen, to provide targeted therapy and specific management and prevent the development of drug resistance. Specific identification will provide the patterns of microbes causing VVI in HIV seropositive patients and those which may colonise and remain latent in the HIV seropositive women and may subsequently proliferate and cause fulminant infection. To improve the diagnostic value of syndrome management, microscopy and culture are valuable tools for determining the pathogens for VVI in resource-poor settings [10]. ART may reduce the incidence of STI among the HIV seropositive women and syndromic management approach for developing countries is also a way towards reducing the burden of HIV/STI co-infections in the community [11,12]. However, large asymptomatic populations with high-risk behaviour remain a source for transmitting agent and continue to be a threat to the community. Hence, this study was undertaken to identify the occurrence of vaginitis in symptomatic and asymptomatic seropositive women and also to find the precise aetiological agent to decide the appropriate treatment for the women attending the STI clinic of a tertiary care hospital in Delhi, India.
Materials and Methods
This cross-sectional, observational, and analytical study was conducted in the Department of Microbiology, Gynaecology and Obstetrics, and the STI clinic of the Department of Dermatology of a tertiary care hospital during a two month period from July 2019 to August 2019. The study was carried out in accordance with the recommendations of National guidelines by National AIDS Control Organisation (NACO) and after obtaining Institutional Human Ethical Committee (IHEC) approval (IEH-HR/2019/39/2R), in accordance with the Helsinki Declaration, with written informed consent from all subjects before collection of samples [13]. A total of 120 HIV seropositive (60 symptomatic for vulvovaginitis and 60 asymptomatic) female patients in the age group of 18-60 years were included in the study. Pregnant and menstruating women or those with a history of diabetes mellitus or smoking were excluded from the study. The samples were collected as per NACO guidelines, i.e., all HIV seropositive women with or without complaints of vaginal discharge were referred from ART Clinic to Gynaecology and Obstetrics OPD and the STI clinic [13]. All the subjects in the study were on HAART and referred to the ART centre of the hospital for receiving ART. After taking precautions, two sterile swabs from each patient were used to collect vaginal secretions from posterior fornix and vaginal walls. The samples were processed for direct microscopy by preparing a wet mount preparation and Gram stain smear of the vaginal secretions. The swabs were cultured on SDA (Hi -media, Mumbai) with Chloramphenicol (0.05 g/L) and Gentamycin (20 mg/L) and Cycloheximide (0.4 mg/L). All inoculated tubes were incubated at 25°C for optimal growth. The yeast positive cultures were further sub-cultured on CHROMagar for identification, and antifungal susceptibility (Fluconazole 25 μg and Voriconazole 10 μg) was performed by disc diffusion method as per CLSI 44A [14].
BV was evaluated by observing the gram stained smear of vaginal secretions under 100X magnification and was scored as per the Nugent system and Amsel scoring system [15,16]. In the calculation of the Nugent score, the number of Lactobacillus spp. (large, Gram-positive rods), Gardnerella spp. (Gram-negative cocco-bacillary organisms), and Mobiluncus spp. (thin, curved Gram-variable rods) morphotypes were evaluated in each oil-immersion microscopic field. By convention, a score of 0–3 was considered normal, a score of 4–6 indicated an intermediate state, and a score of 7-10 was regarded as indicative of BV. Morphotypes were scored as average number per high powered (1000× oil immersion) field.
The Amsel criterion was used for the confirmation of bacterial vaginosis [17]. The four parameters checked to determine the presence or absence of BV included thinned, white, yellow, homogeneous discharge; clue cells on wet mount microscopy; a vaginal fluid pH of over 4.5 when placing the discharge on litmus paper, and release of fishy odour on adding 10% potassium hydroxide (KOH) solution to wet mount (“whiff test”). Three of the four aforementioned criteria must be present to confirm the diagnosis. The wet mount was also scanned for yeast, Trichomonas spp, epithelial cells, parabasal cells and pus cells.
The diagnosis of AV was based on microscopic criteria as per the Donder’s classification [Table/Fig-1] [18]. For scoring purposes, along with the relative number of leucocytes, percentage of toxic leucocytes, background flora and proportion of epitheliocytes, lactobacillary grade under 400x (high-power field) were evaluated as per the [Table/Fig-2,3]. The “AV score” was compiled and calculated as <3 indicating no signs of AV; AV score 3 or 4 indicating light AV; a score of 5 or 6 is moderate AV and ≥7 suggestive of severe AV [18].
Microbiological assessment of aerobic vaginitis as per Donders classification.
| AV score | 0 | 0 | 1 | 2 |
|---|
| Lactobacillary grades | I | II-a | II-b | III |
| Numerous pleomorphic Lactobacilli; no other bacteria | Mixed flora, but predominantly Lactobacilli | Mixed flora, but the proportion of Lactobacilliseverely decreased because of an increased number of other bacteria | Lactobacilli severely depressed or absent because of overgrowth of other bacteria. |
| Number of leukocytes | <10/hpf | <10/hpf | >10/hpf and; <10/epithelial cell | >10/epithelial cell |
| Proportion of toxic leucocytes | None or sporadic | None or sporadic | ≤ 50% of leucocytes | ≥ 50% of leucocytes |
| Proportion of parabasal epitheliocytes | None or <1% | None or <1% | ≤10% | >10% |
hpf- high power field
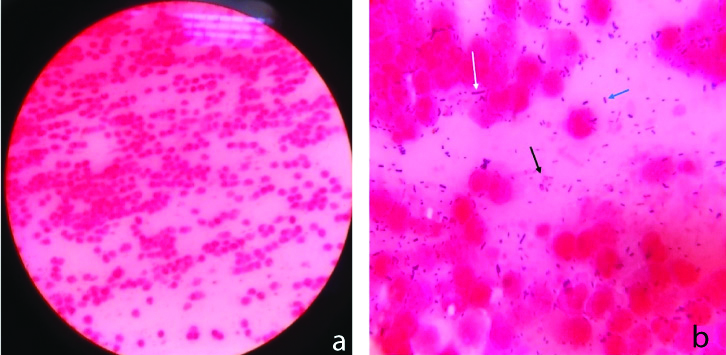
Gram-stained smear of vaginal discharge; a) Plenty of leukocytes (>10 leucocytes/ epithelial cell) under 40X magnification of light microscope; b) Gram-negative bacilli (black arrow); Gram-positive cocci in pairs (blue arrow) and very few Lactobacilli which are thick Gram-positive bacilli (white arrow) with plenty of leucocytes under 100X magnification of a light microscope.
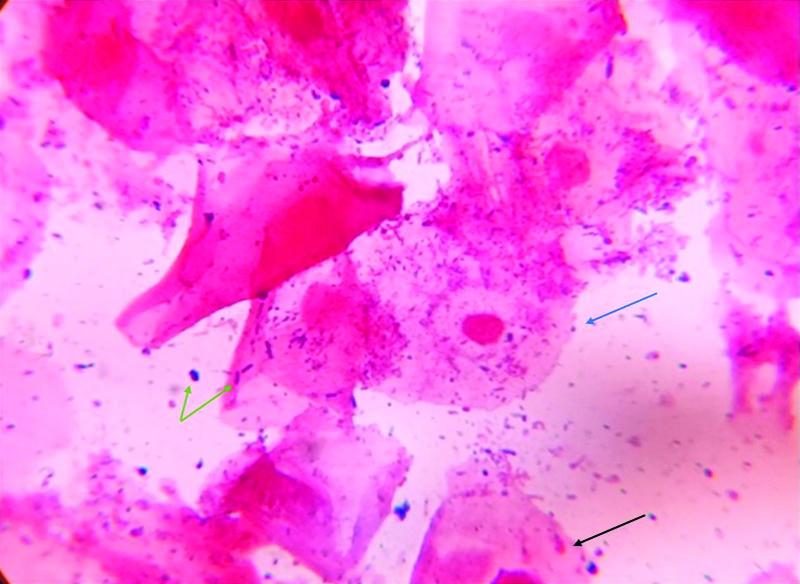
Gram-stained smear of vaginal discharge under 100X magnification of a light microscope, depicting vaginal squamous epithelial cells (black arrow), clue cells (blue arrow) and mixed flora (green arrow).
The antifungal susceptibility testing was done as per CLSI 44 A (Yeast) by disc diffusion method for Fluconazole (25 μg) and Voriconazole (10 μg) [14,19]. Growth of Candida spp on SDA was checked for purity and viability at 25°C for 24 hours. Five colonies of 1 mm size were picked up from 24-hour culture. These colonies were suspended in 5 mL of 0.145 mol/L saline (0.85% saline). The suspension was vortexed for 15 seconds and adjusted to 0.5 McFarland turbidity at 530 nm to yield 1×106 or 5×106 cells/mL to give semi confluent growth. After 15 minutes of adjusting turbidity, a sterile cotton swab was taken, dipped in suspension and pressed to remove excess fluid. Dry surface of Mueller Hinton Agar (MHA) was inoculated by evenly streaking over the entire surface of the agar and rotating all around and the rim of the agar. Plates were put to dry for five minutes and within 15 minutes; the discs were placed on the plate and incubated at 35°C for incubation. Circular zone of inhibition was noted after 24 hours of growth. Control strains of ATCC C.albicans 90028 and ATCC C.parapsilosis 22019 were used for antifungal susceptibility test.
Statistical Analysis
The collected data were compiled and entered into Microsoft Excel spreadsheet. The descriptive analysis was done to interpret and analyse the findings. Chi-square tests were used where applicable to compare differences between proportions. A p-value <0.05 was considered as statistically significant.
Results
Out of 120 HIV seropositive female patients screened for VVI, 60 symptomatic and 60 asymptomatic for vulvovaginitis were included in the study. The demographic profile of symptomatic and asymptomatic seropositive women enrolled in the study, which included age distribution, education, occupation, barrier usage and age of first sexual exposure is shown in [Table/Fig-4]. Majority of the symptomatic and asymptomatic women were in the age group of 18-30 years (45%). Thirty-nine percent (39.16%) symptomatic and asymptomatic women were illiterate, and only 10.83% were graduates. Most were housewives by occupation (58.33%), 11.66% were labourers, 1.66% had permanent jobs, and 15% were students, while 13.33% were self-employed. Also, amongst sexual partners of symptomatic seropositive women, only five (8.33%) had complaints of discomfort and discharge as genital complaints (data not shown).
Profile of symptomatic and asymptomatic HIV seropositive women.
| Symptomatic | Asymptomatic |
|---|
| N=60 | % | N=60 | % |
|---|
| Demographic profile |
| Age distribution* |
| 18-30 years | 37 | 61.67% | 17 | 28.33% |
| 31-45 years | 18 | 30.00% | 27 | 45.00% |
| 46-60 years | 5 | 8.33% | 16 | 26.67% |
| Education level |
| No education | 23 | 38.33% | 24 | 40.00% |
| Till 8th class | 17 | 28.33% | 14 | 23.33% |
| 9th-10th class | 8 | 13.33% | 7 | 11.67% |
| 11th-12th | 7 | 11.67% | 6 | 10.00% |
| Graduate | 5 | 8.33% | 8 | 13.33% |
| Postgraduate | 0 | 0.00% | 1 | 1.67% |
| Occupation |
| Housewife | 39 | 65.00% | 31 | 51.67% |
| Labourer | 8 | 13.33% | 6 | 10.00% |
| Job | 1 | 1.67% | 1 | 1.67% |
| Student | 5 | 8.33% | 13 | 21.67% |
| Self-employed | 7 | 11.67% | 9 | 15.00% |
| Barrier used |
| Yes | 36 | 60.00% | 33 | 55.00% |
| No | 24 | 40.00% | 27 | 45.00% |
| Age of 1st sexual exposure |
| Not yet | 1 | 1.67% | 1 | 1.67% |
| 10-15 y | 8 | 13.33% | 15 | 25.00% |
| 16-20 y | 39 | 65.00% | 31 | 51.67% |
| 21 y and more | 12 | 20.00% | 13 | 21.67% |
| Clinical profile |
| Bacterial vaginosis (by Nugent score) |
| Positive (7 or >7) | 42 | 70.00% | 33 | 55.00% |
| Intermediate (4-6) | 12 | 20.00% | 19 | 31.66% |
| Negative (0-3) | 6 | 10.00% | 8 | 13.33% |
| Bacterial vaginosis (by Amsel’s score) |
| Positive | 29 | 48.33% | 34 | 56.66% |
| Negative | 31 | 51.66% | 26 | 43.33% |
| Aerobic vaginitis** |
| Positive | 6 | 10.00% | 20 | 33.33 |
| Negative | 54 | 90.00% | 40 | 66.66 |
| Scoring for aerobic vaginitis (Donder’s classification) |
| Mild (3-4 score) | 6 | 10.00% | 13 | 21.66% |
| Moderate (5-6 score) | 0 | 0.00% | 7 | 11.66% |
| Severe (7-10 score) | 0 | 0 | 0 | 0 |
| CD4 count (cells/mm3) |
| <200 | 6 | 10.00% | 5 | 8.33% |
| 200-499 | 16 | 26.66% | 21 | 35.00% |
| ≥500 | 38 | 63.33% | 34 | 56.66% |
| Mycological profile |
| Total culture-positive yeast species |
| Candida albicans | 12 | 20.00 | 10 | 16.66 |
| Candida glabrata | 2 | 3.33 | 2 | 3.33 |
| Candida parapsilosis | 1 | 1.66 | 1 | 1.66 |
| Candida tropicalis | 1 | 1.66 | 0 | 0.00 |
| Candida auris | 0 | 0.00 | 1 | 1.66 |
| Candida kefyr | 1 | 1.66 | 0 | 0.00 |
| Candida krusei | 0 | 0.00 | 1 | 1.66 |
| Yeast species with BV (Nugent’s 4-10) |
| Candida albicans | 11 | 18.33% | 9 | 15.00% |
| Non-Candida albicans | 5 | 8.33% | 4 | 6.66% |
* p-value <0.001; ** p-value=0.002
[Table/Fig-5] describes the characteristics of vaginal discharge in symptomatic women. Majority of symptomatic HIV seropositive females (60.00%) presented with white and watery discharge. In the study, 34 (32.07%) symptomatic HIV seropositive females with BV (Nugent score of 4-10) presented with a white, watery type of vaginal discharge as shown in [Table/Fig-6]. Fifty-two (49.05%) asymptomatic HIV seropositive females had positive Nugent score for BV as identified by altered vaginal flora found on microscopic examination of vaginal smear shown in [Table/Fig-6]. On examining the severity score, 42 (70%) symptomatic females had severe bacterial vaginosis as per Nugent score while 33 (55%) asymptomatic with bacterial vaginosis had a score more than 7 as per [Table/Fig-4]. The overall percentage of BV was high in symptomatic as well as asymptomatic HIV seropositive women at 90% and 86.66%, respectively. On using the Amsel score, 63/120 (52.50%) samples were positive for BV shown in [Table/Fig-4]. On further assessing the Gram-stained smears of the vaginal samples, AV was significantly positive in asymptomatic HIV seropositive females (33.33%) as compared to the symptomatic females (10%) (p < 0.05). The severity of AV was assessed using the Donders classification, as shown in [Table/Fig-4].
Nature of vaginal discharge reported by symptomatic HIV seropositive females.
| Nature and type of discharge | Symptomatic HIV seropositive females (N=60) |
|---|
| N | % |
|---|
| White, watery | 36 | 60.00% |
| White, thick | 22 | 36.67% |
| Yellow, watery | 1 | 1.67% |
| Watery (blood) | 1 | 1.67% |
Comparison of bacterial vaginosis presentation in both asymptomatic and symptomatic HIV seropositive vaginal discharges.
| Nature and type of discharge | BV positive (Nugent score 4-10) (N=106) |
|---|
| N | % |
|---|
| Symptomatic | white, watery | 34 | 32.07 |
| white, thick | 18 | 16.98 |
| yellow, watery | 1 | 0.94 |
| watery (blood) | 1 | 0.94 |
| Asymptomatic | 52 | 49.05 |
Another interesting observation was that VVC was found almost equally in both symptomatic and asymptomatic HIV seropositive females 32/120 (26.66%) as per [Table/Fig-4]. Candida albicans was the predominant isolated pathogen. All the Candida isolates were sensitive to both Fluconazole and Voriconazole (with zone diameter >19 mm and >17 mm for Fluconazole and Voriconazole, respectively). Co-infection of bacterial Vaginosis (Nugent score of 4-10) and candidiasis, was observed in 29/120 (24%) samples in symptomatic and asymptomatic HIV seropositive females.
CD4 count ≥500 cells/uL was observed in 38 (63.33%) symptomatic and 34 (56.66%) asymptomatic HIV seropositive females on ART, as shown in [Table/Fig-4]. On comparing the CD4+ count with clinical symptoms, it was observed that HIV seropositive females on ART with a count of ≥500 cells/μL had bacterial vaginosis (58.49%), candidiasis in 65.62% and co-infection with both BV and VVC in 65.5% which was higher when compared to lower CD4 counts [Table/Fig-7].
Distribution of CD4 count in the different aetiological presentation of vaginosis.
| CD-4 count | Bacterial vaginosis positive (by Nugent’s) (N=106) | Bacterial vaginosis negative (N=14) | Yeast positive (N=32) | Co-infection (BV+Yeast) (N=29) |
|---|
| N | % | N | % | N | % | N | % |
|---|
| <200 | 10 | 9.43% | 1 | 7.14% | 4 | 12.50% | 4 | 13.79% |
| 200-499 | 34 | 32.07% | 3 | 21.42% | 7 | 21.87% | 6 | 20.68% |
| ≥500 | 62 | 58.49% | 10 | 71.42% | 21 | 65.62% | 19 | 65.51% |
Discussion
The ecological existence of normal microbial flora at various anatomical sites defines their role in maintaining a barrier and preventing the establishment of infection by pathogens. Any immunological disturbance or loss of innate defence mechanism elicits a paradigm shift of commensal flora to behave as pathogens. This study highlights the association of VVIs in symptomatic HIV seropositive women on ART (average duration of 5 years) compared with the vaginal micro-flora in asymptomatic HIV seropositive women attending OPD at a tertiary care hospital, Delhi, India. High-risk sexual practices and multiple partners are favourable factors for acquiring VVI in women. Furthermore, the association of genital complaints in the partners of the symptomatic HIV seropositive females was also observed in 8.33% cases in present study, indicating robust transmissibility in the absence of barrier protection.
Bacterial vaginosis was the predominant manifestation in 88.33% of HIV seropositive females followed by VVC in 26.66% though the mixed infection was also observed in 24.16% of women. Very often, a co-infections of BV and VVC may remain undetected unless a microscopic examination of vaginal smear and culture for candidiasis is done in a laboratory to establish dual infection. While the contribution of BV or AV in the progression of vaginitis in HIV seropositive women is incompletely known. The predominance of Lactobacilli in the lower genital tract of the healthy female is in contrast to the microbial diversity observed in BV which is responsible for the overwhelming proliferation of microbes of different genera of Gram-positive and Gram-negative organisms like Gardnerella vaginalis, Prevotella sp., Bacteroides sp., Peptostreptococcus sp., Mycoplasma hominis and Mobiluncus sp. [5,20,21]. Though earlier studies suggest the occurrence of BV in up to 24% symptomatic HIV seronegative women, often lower rates are reported in women who are not sexually active [22,23]. Rather, approximately 50% of females with BV may even remain undetected [24]. It is well documented that women with BV are more likely to transmit HIV to males [25]. Taha TE et al., showed the association of BV with increase in the risk of HIV acquisition in their longitudinal follow-up study [26]. Many studies performed in Thailand, Uganda, and Malawi, suggested that women with BV had an increased incidence of HIV infection but could not prove cause and effect of relationship between BV and HIV infection [26-28].
In present study, we prospectively studied HIV seropositive women with complaints of vaginal discharge with no other risk factors like smoking or diabetes mellitus. Though 90% of symptomatic HIV reactive women had BV, 86.66% asymptomatic HIV reactive females too were detected with BV. The serendipitous finding of BV in asymptomatic HIV seropositive women raises concern. As it being a time-dependent condition, if undetected, these women will eventually become a reservoir of diverse microbiota and provide a favourable environment for HIV replication [29]. The potential impact of BV in HIV transmission to partners is a major link in the acquisition of other STIs, thus posing a threat to the failure of HIV and STI control programme.
The severity score of BV as per Nugent scoring system was high in 70% in HIV seropositive symptomatic females compared to the asymptomatic females, Amsel criteria could only detect 48.33% of BV in symptomatic HIV seropositive females. Though Amsel criteria is easy to achieve its accuracy, underscores its utility to classify BV accurately compare to Nugent scores which was similar to the studies by Bansal R et al., and Sha BE et al., [30,31].
Besides the classical STIs, AV has been defined as an inflammatory variant of vaginal symbiosis associated with purulent discharge and local inflammation identified by Gilbert Donders [18]. A vaginal condition considered being distinct from BV, it remains underdiagnosed and escapes detection due to its indistinct clinical presentation. Associated with distinct clinical presentations and management, AV is characterised by intense vaginal inflammation with an increased number of toxic leucocytes. Surprisingly, in the present study, higher aerobic vaginitis (33.33%) in asymptomatic patients was observed compared to symptomatic HIV seropositive females. Concomitant existence of BV in these patients can masquerade the clinical presentation as BV may be difficult to differentiate in mixed AV infections. The asymptomatic HIV seropositive women harbour a diverse population of microbes comprising of Escherichia coli, Group B Streptococci, Enterococcusfecalis and Staphylococcus aureus which are detectable only on the Gram-stained smear. Hence in resource-poor settings, Gram-stained smears still accomplish as the gold standard tool to characterise BV, VVC and mixed infection for the administration of appropriate treatment and prevent relapses in these patients.
The transformation of C. albicans from a harmless commensal to a virulent pathogen has been attributed to a dysfunctional host defence system and the interplay of fungal virulence determinants [32]. The manifestation of VVC in symptomatic HIV seropositive females or colonisation of yeast in asymptomatic HIV seropositive females are still the well-established indicators of active transmission of HIV infection despite being on ART with a CD+4 count of >500 cells/uL. Though BV has been noted to be the most prevalent vaginal infection in adult women worldwide especially in sexually active women, co-infection of yeast with BV (≥7) which was observed in 26.66% of symptomatic and 21.66% asymptomatic HIV seropositive females in present study is similar to other studies [6]. Scant literature on mixed vaginal infection (BV and VVC) state that treatment of BV component can only exacerbate VVC if left untreated and remain a potential source of infection [33,34].
Thus, the dynamics of vaginitis among HIV/AIDS patients may result in the emergence of pathogenic microbial species resistant to antimicrobial agents leading to chronicity [35]. The knowledge about the effectiveness of ART in treating vaginal infections in HIV-infected women is still limited [36]. Despite on ART and CD4 count >500 cells/μL., 58.49% HIV women in present study harboured BV. Hence, we hypothesise that providing treatment to symptomatic as well as asymptomatic cases of vaginitis in HIV/AIDS patients would considerably reduce the burden of VVI [9]. So, it is important to evaluate the changing rate of these common vaginal infections in the context of the increased use of ART.
Hence, a need for early and accurate detection of BV, AV and VVC and set up point-of-care tests for early diagnosis, may provide better therapeutic management and prevention of relapses thereby minimising the occurrence of antibiotic resistance.
Limitation(s)
The observational study was limited to the analysis of VVI in a small target population for two months. Further studies on a larger study group with repeat follow-up, and suitable point-of-care tests can provide evidence of probable pathogens or reservoirs of microbes causing persistence of infections and provide appropriate treatment and minimising unnecessary therapeutic cover of VVI.
Conclusion(s)
Despite ART, attendees manifested significantly with BV infection in symptomatic as well as in the asymptomatic HIV seropositive females. VVC due to C. albicans was predominant in both the groups. Co-infection was the highlight of this study as dual infections of BV and AV remain under-diagnosed as per STI syndromic management. The clinicians need to identify AV as a separate entity as treatment modalities are essentially different. Intravaginal antimicrobials, steroids are the line of treatment for AV as per the degree of inflammation and severity of the lesion, though ideal duration has not yet been established. To consider the asymptomatic HIV seropositive women with BV or VVC and AV as a potential reservoir of pathogens, screening protocols for this cohort of the population may have a beneficial impact in reducing STI and prevent the emergence of drug-resistant genital microbial flora.
hpf- high power field
* p-value <0.001; ** p-value=0.002