Severe Pulmonary Air Leak Complicating Neonatal Resuscitation
Supriya1, Perumalla Bhavani Deepthi2, S Giridhar3
1 Postgraduate, Department of Paediatrics, Chettinad Hospital and Research Institute, Chennai, Tamil Nadu, India.
2 Postgraduate, Department of Neonatology, Chettinad Hospital and Research Institute, Chennai, Tamil Nadu, India.
3 Associate Professor, Department of Neonatology, Chettinad Hospital and Research Institute, Chennai, Tamil Nadu, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. S Giridhar, Associate Professor, Department of Neonatology, Chettinad Hospital and Research Institute, Chennai, Tamil Nadu, India.
E-mail: giridharsethu@gmail.com
Air leaks are an important respiratory morbidity in neonates and can result from positive pressure breaths administered at birth. The presentations can vary from being asymptomatic to severe respiratory distress and hypoxaemia. This report is about a term boy who presented with respiratory distress immediately after birth, following resuscitation. He also had a diffuse swelling in the nape of the neck and interscapular region with crepitus on palpation. Serial chest radiographs initially revealed a pneumomediastinum and a subcutaneous emphysema, followed later by a right-sided pneumothorax. After chest drain insertion and supportive care, the air leaks resolved, with no residual complications. Severe air leaks with multi-site air tracking can rarely complicate positive pressure administration at birth, requiring timely intervention to optimise outcomes.
Chest drain, Respiratory morbidity, Swelling
Case Report
A baby boy was born in the hospital to a G3 P2 L1 A1 mother. The mother had an uneventful antenatal period and developed labour pains at 38 weeks. After full cervical dilatation, the second stage of labour was prolonged and the foetal monitoring was done by Doppler Ultrasound which showed a dip in the foetal heart rate. The baby was delivered vaginally assisted by outlet forceps and was depressed at birth, requiring resuscitation. He was given bag and mask ventilation but was not intubated. APGAR scores were 3/10 at 1 minute and 7/10 at five minutes. Baby soon developed respiratory distress and was started on supplemental hood oxygen and then was shifted to the Neonatal Intensive Care Unit.
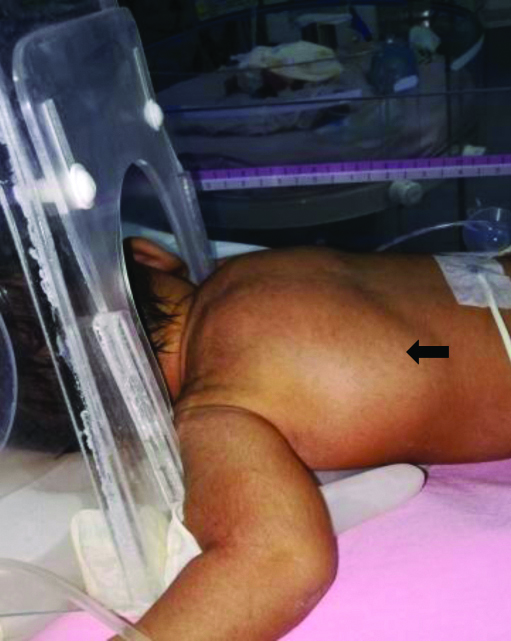
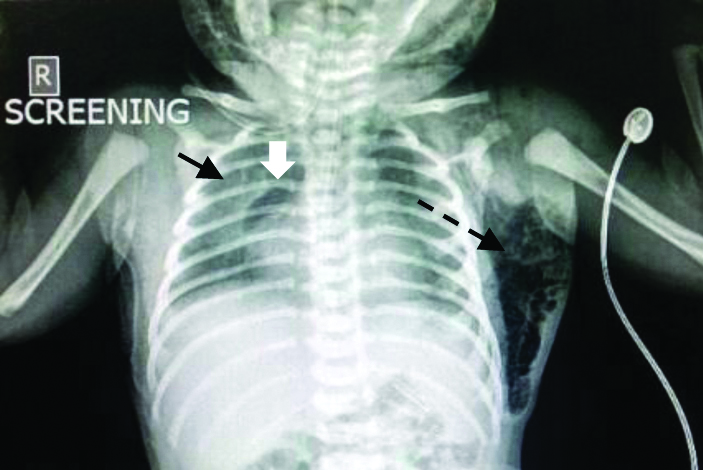
At admission, the baby had the following vital signs: respiratory rate-80/min; heart rate-160/min; axillary temperature-36.3°C, blood pressure-56/35, mean-41 mmHg, SpO2-93%, right wrist receiving hood oxygen of 3 L/min. There was significant intercostal and subcostal chest indrawing. A diffuse swelling was noticed in the nape of the neck extending into the bilateral scapular regions, which on palpation showed some crepitus [Table/Fig-1]. There was no overlying skin discolouration, induration or tenderness. The baby was otherwise active and had a normal cardiovascular, abdominal and neurological examination. Arterial blood gas analysis showed respiratory acidosis (pH-7.23, pO2-68 mmHg, pCO2-49 mmHg), but the blood counts and chemistry were otherwise normal. The first chest radiograph showed a right pneumomediastinum (spinnaker sail sign) and left sided subcutaneous emphysema [Table/Fig-2].
Clinical photograph showing the subcutaneous air collection presenting as a swelling in the right scapular region (Black arrow).
X-ray showing halo around right heart border (white arrow) and lateral displacement and delineation of the right thymic lobe called the spinnaker sail sign (black arrow), findings suggestive of pneumomediastinum. A subcutaneous emphysema is also seen as a radiolucency within the soft tissue in the left flank (dotted arrow).
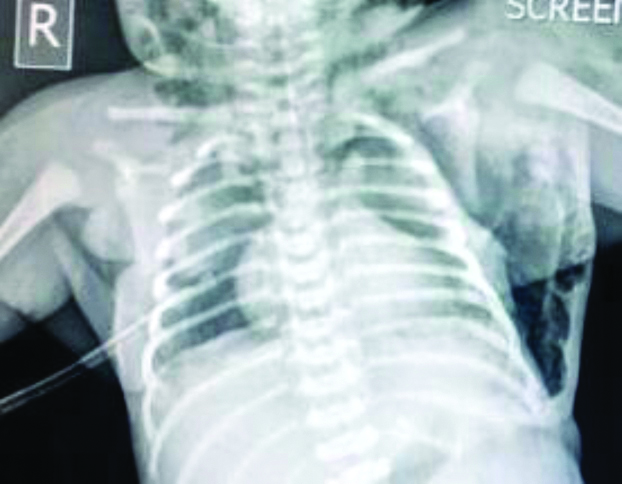
One hour after admission, there was worsening of respiratory distress and a repeat chest x-ray showed right-sided pneumothorax along with the pneumomediastinum and hence, a right chest drain was placed [Table/Fig-3]. Post-chest drain insertion, the respiratory distress improved and the subcutaneous emphysema regressed over the next 24 hours.
Showing right-sided pneumothorax drained using a chest tube. The pneumomediastinum (sail sign) and subcutaneous emphysema are also seen.
Serial chest radiographs showed resolution of air leaks and the chest drain was clamped and removed by 48 hours of life. The baby was weaned to room air by 52 hours of admission. Flexible fibre-optic bronchoscopy was done using a 2.8 outer diameter (OD scope), to rule out any airway trauma, upper oesophageal injuries and malformations, and it was normal. By the time of discharge, on day seven of life, the baby was feeding well, neurologically normal and gaining weight adequately with no respiratory distress.
Discussion
Respiratory distress in a term infant has many causes, ranging from transient tachypnoea to newborn to structural lung diseases. Air leaks are an important neonatal respiratory morbidity, in both term and preterm infants. If not recognised earlier, air leaks may become severe, resulting in respiratory failure and death. In the National Neonatal Perinatal Database 2002-2003, air leaks were found in 0.1% of intramural births and 1.6% of extramural admissions, and were the fourth most common respiratory morbidity in intramural neonates [1]. Multi-site pulmonary air collections are a rare presentation, but have been reported in neonates previously [2,3].
Air leaks could be spontaneous or induced. Spontaneous air leaks may occur as a result of the increase in self-generated alveolar distending pressure, rupturing the alveoli. This can happen at birth or during crying episodes [4]. Majority of spontaneous air leaks result in mild interstitial air leak or pneumomediastinum and are asymptomatic. Induced air leaks, although less common are more likely to be symptomatic. In this case, the air leak was induced by the positive pressure breaths given during resuscitation. Bag and mask ventilation at birth has been well-recognised as a major risk factor for air leaks in neonates [5]. The variable distending pressure generated by an ambu bag could result in alveolar rupture and this is more common in low compliance conditions like a fluid-filled lung at birth or respiratory distress syndrome. Another important reason for induced air leaks is mechanical ventilation with a reported incidence ranging from 20 to 26% [6-8].
Pneumomediastinum results from air tracking along the peribronchovascular sheath into the hilum of the lung, a phenomenon described by Macklin CC in 1939 [9]. The dissection of free air may not be confined solely to the mediastinum. Zylak CM et al., noted that the mediastinum communicates with the vascular sheaths in the neck and this could have caused the cervical and interscapular emphysema, in the index patient [10].
A simple chest radiograph is an important tool for the diagnosis of pulmonary air leaks. Pneumomediastinum is seen as a ring-like translucency around the lateral borders of the heart in the anteroposterior views. It should be differentiated from pneumopericardium by the absence of translucency in the inferior border of the heart. Another well-described radiological sign of pneumomediastinum is the delineation and displacement of the thymic lobes by the air, resembling the headsail of a boat, and called the “spinnaker sail sign” or “angel wing sign” [11]. Both these signs are well seen in the chest radiographs of the case. Subcutaneous emphysema is more often diagnosed clinically by the characteristic crackling sensation felt during pressure on the swelling. The extent can then, be known by radiographs showing, subcutaneous emphysema as radiolucent areas in the soft tissues.
The combined presentation of pneumomediastinum and subcutaneous emphysema has previously been reported due to trauma to the upper airway structures like trachea during the process of intubation [12]. It has also been previously reported after oesophageal rupture due to repeated feeding tube insertions [13]. In order to rule out these aetiologies, flexible fibreoptic bronchoscopy was done in this case and none were noted.
Treatment of neonatal air leaks is usually watchful expectancy with most leaks spontaneously resolving over a period of time. In the present case, the presence of severe air leaks like pneumothorax and subcutaneous emphysema, with pneumomediastinum, necessitated insertion of the right side intercostal chest drain to evacuate the air. Corsini I and Dani C had also reported that the severe air leaks were the ones that usually required intervention like chest drains and the milder cases resolved spontaneously [14].
Conclusion(s)
Pneumomediastinum progressing to pneumothorax and subcutaneous emphysema is a rare presentation, and can result from positive pressure breaths administered after birth to a neonate. Watchful expectancy with timely insertion of chest drains is necessary to optimise outcomes.
Author Declaration:
Financial or Other Competing Interests: None
Was informed consent obtained from the subjects involved in the study? Yes (from parents)
For any images presented appropriate consent has been obtained from the subjects. Yes (from parents)
Plagiarism Checking Methods: [Jain H et al.]
Plagiarism X-checker: Jun 15, 2020
Manual Googling: Aug 13, 2020
iThenticate Software: Sep 15, 2020 (4%)
[1]. Report of National Neonatal Perinatal Database (NNPD) 2002-2003. Available from: http://www.newbornwhocc.org/pdf/nnpd_report_2002-03.pdf. Accesssed July 30, 2020 [Google Scholar]
[2]. Hatanaka D, Nakamura M, Kusakari M, Takahashi H, Nakamura T, Kamohara T, Neonatal subcutaneous emphysema and pneumomediastinum soon after birthPediatr Int 2016 58(6):541-42.10.1111/ped.1293327322866 [Google Scholar] [CrossRef] [PubMed]
[3]. Nieto-Barcelo JJ, Costa-Roig A, Parra-Llorca A, Escrig R, Enfisema subcutáneo neonatal [Neonatal subcutaneous emphysema]An Pediatr (Barc) 2019 90(4):252-253.doi:10.1016/j.anpedi.2018.03.011. Available from: https://pubmed.ncbi.nlm.nih.gov/29691133/10.1016/j.anpedi.2018.03.01129691133 [Google Scholar] [CrossRef] [PubMed]
[4]. Ashmore PG, Spontaneous pneumothorax in the newbornCan Med Assoc J 1965 92(7):309-11. [Google Scholar]
[5]. Ngerncham S, Kittiratsatcha P, Pacharn P, Risk factors of pneumothorax during the first 24 hours of lifeJ Med Assoc Thai 2005 88(Suppl 8):S135-41. [Google Scholar]
[6]. Baumer JH, International randomised controlled trial of patient triggered ventilation in neonatal respiratory distress syndromeArch Dis Child Fetal Neonatal Ed 2000 82(1):F5-F10.10.1136/fn.82.1.F510634832 [Google Scholar] [CrossRef] [PubMed]
[7]. Bösche C, Genzel-Boroviczény O, Hepp H, Knitza R, Versmold H, Roos R, Mortality, mode of delivery, pneumothorax and intracranial hemorrhage in 859 extremely premature newborn infants between 1984-1992Geburtshilfe Frauenheilkd 1996 56(6):322-27.10.1055/s-2007-10232388766491 [Google Scholar] [CrossRef] [PubMed]
[8]. Malek A, Afzali N, Meshkat M, Yazdi NH, Pneumothorax after mechanical ventilation in newbornsIran J Pediatr 2011 21(1):45-50. [Google Scholar]
[9]. Macklin CC, Transport of air along sheaths of pulmonic blood vessels from alveoli to mediastinum: Clinical implicationsArch Intern Med 1939 64:913-26.10.1001/archinte.1939.00190050019003 [Google Scholar] [CrossRef]
[10]. Zylak CM, Standen JR, Barnes GR, Zylak CJ, Pneumomediastinum revisitedRadiographics 2000 20(4):1043-57.10.1148/radiographics.20.4.g00jl13104310903694 [Google Scholar] [CrossRef] [PubMed]
[11]. Correia-Pinto J, Henriques-Coelho T, Images in clinical medicine. Neonatal pneumomediastinum and the spinnaker-sail signN Engl J Med 2010 363:214510.1056/NEJMicm100246221105796 [Google Scholar] [CrossRef] [PubMed]
[12]. Ammari A, Jen A, Towers H, Joseph Haddad Jr J, Wung JT, Berdon WE, Subcutaneous emphysema and pneumomediastinum as presenting manifestations of neonatal tracheal injuryJ Perinatol 2002 22:499-501.10.1038/sj.jp.721075812168130 [Google Scholar] [CrossRef] [PubMed]
[13]. Lee SH, Kim JK, A case of neonatal pneumomediastinum with subcutaneous emphysema suspected to be caused by pharyngoesophageal injuryNeonatal Med 2020 27(1):21-25.10.5385/nm.2020.27.1.21 [Google Scholar] [CrossRef]
[14]. Corsini I, Dani C, Pneumomediastinum in term and late preterm newborns: What is the proper clinical approach?Matern Fetal Neonatal Med 2015 28(11):1332-35.10.3109/14767058.2014.95392425178357 [Google Scholar] [CrossRef] [PubMed]