Omphalomesenteric Duct anomalies are a rare group of congenital malformations of the primitive yolk sac [1,2]. OMD anomalies are found in 2% of population [3]. The spectrum of OMD anomalies includes Meckel’s diverticulum, Meckel’s diverticulum with fibrous band, Patent Vitellointestinal Duct (PVID) and others (sinus tract, umbilical polyp and cyst) [4]. Meckel’s diverticulum is the commonest OMD anomaly with incidence of 83% [4]. Incidences of Meckel’s diverticulum with fibrous band and PVID are 10% and 6%, respectively [4]. Incidence of other anomalies like sinus tract, umbilical polyp (mucosal remnant) and cyst is less than 1% [4].
The OMD provides a communication between primitive yolk sac and the midgut of the developing embryo [2] and serves to transfer nutrients from the yolk sac to the developing embryo. The OMD resembles an extension of superior mesenteric artery towards and beyond the umbilicus and follows the axis of the herniating midgut loop. As the foetus grows, the foetal intestines gradually separate from the yolk sac leaving the ductal communication with the yolk sac itself [1]. This ductal communication gradually involutes around 5th to 9th week of gestation beginning at the ileum [1]. Partial or complete failure of this involution leads to OMD anomalies.
Non-involution of the OMD may present with varied clinical features such as umbilical discharge, umbilical infection, umbilical polyp, per rectal bleed, abdominal pain, intestinal obstruction and rarely, acute abdomen [5,6]. OMD remnants may sometime remain asymptomatic throughout life [7,8]. Complications ranging from haemorrhage and obstruction in younger patients to malignancy in older patients have been reported [9]. Although mortality related with OMD anomalies have declined over a period of time from 0.01% to 0.001%, higher mortality has been reported in the paediatric age group [10].
The aim of the current study was to study the spectrum and management of symptomatic OMD anomalies in children at a rural tertiary care centre.
Materials and Methods
This was a retrospective study done at Uttar Pradesh University of Medical Sciences, Saifai, Etawah, a tertiary care centre located in rural part of Northern India and primarily catering to rural population. The study was conducted jointly by the Departments of Paediatric Surgery and Pathology of the University. Medical records of all children less than 15 years of age who were diagnosed with symptomatic OMD anomalies and were managed surgically between January 2016 to December 2019 (four years) were carefully examined and data collected from them after clearance from the Institute Ethics Committee (IEC no 2019/32) in October 2019. Data of children who underwent surgery for OMD anomalies beyond the mentioned time period were excluded from the study. Symptomatic OMD anomalies were those which presented with acute abdomen, intestinal obstruction and umbilical abnormalities. The parameters recorded were age, sex, symptomatology, clinical presentation, intraoperative findings, surgical procedure, complications that developed during the early postoperative period, length of hospital stay and HPE reports of the specimens excised.
Statistical Analysis
Data was summarised in form of proportions and frequency tables for categorical variables. Data were analysed using SPSS software (Statistical Package for the Social Sciences, version 21.0.0.0. Chicago, Ill, USA). The p-values were computed for categorical variables using the Chi-square test and Fisher’s-exact test depending on the size of the data set. The p-value <0.05 was considered statistically significant.
Results
The Department of Paediatric Surgery performed surgeries on 644 children during the study period. Of these 644, 40 were children with symptomatic OMD anomalies who were managed surgically during the study period and accounted for 6.21% of the total number of surgeries. Of these 40 children, 29 were boys (72.5%) and 11 were girls (27.5%) with male-female ratio of 2.6:1. The mean age of the cohort was 41.77 months (2 months -131 months) and median of 24 months. Seventeen out of 40 children (42.5%) were less than 1 year of age at the time of surgery. All the cases were sporadic. No significant associated anomaly was recorded in this cohort.
Most common presenting complaints for which the parents sought medical assistance were related to either of the following three: intestinal obstruction (n=9 of 40; 22.5%), acute abdomen (n=11 of 40; 27.5%) and umbilical abnormalities (20 of 40; 50%). The incidence of umbilical abnormalities was higher in infancy and was statistically significant (p=0.0039) [Table/Fig-1].
Clinical presentation in infancy and post infancy group.
| Age of presentation | UA | AA | IO | Total |
|---|
| Infancy (up to 1 year) | 13 | 2 | 2 | 17 |
| Children 1-15 year | 7 | 9 | 7 | 23 |
| Total | 20 (50%) | 11 (27.5%) | 9 (22.5%) |
| 40 | p-value | 0.0039* | 0.0553 | 0.162 |
UA: Umbilical Abnormalities; AA: Acute Abdomen; IO: Intestinal Obstruction
p<0.05* statistically significant (Chi-square test)
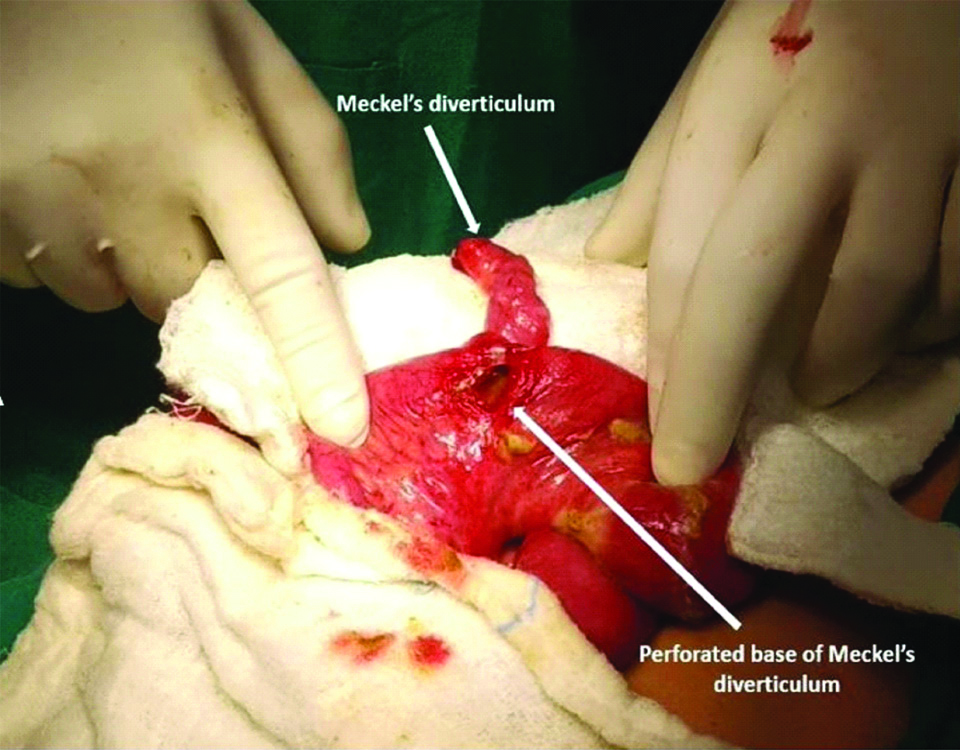
Meckel’s diverticulum was observed in 20 patients (11 patients with acute abdomen, 9 patients with intestinal obstruction and none of the cases with umbilical abnormalities). Of these, Meckel’s diverticulum was perforated [Table/Fig-2] in 11 (11 of 20; 55%) and all such cases presented with acute abdomen. Of the 9 children who presented with intestinal obstruction, seven patients had Meckel’s diverticulum with an obstructing band [Table/Fig-3] and two patients had intussusception with Meckel’s diverticulum acting as a pathological lead point.
Clinical photograph of Meckel’s diverticulum with perforation at its base.
Clinical photograph of Meckel’s band.

Out of the 20 patients who presented with umbilical abnormalities, umbilical polyp was noted in six patients (30%), PVID [Table/Fig-4] in 12 patients (60%) and infected umbilical cyst in two patients (10%) [Table/Fig-5].
Clinical photograph of Patent Vitellointestinal Duct (PVID).

Clinical presentations with intraoperative findings.
| Intraoperative finding | Clinical presentations |
|---|
| UA n (%) | AA n (%) | IO n (%) |
|---|
| Meckel’s with band | 0 | 0 | 7 (77.77%) |
| Meckel’s base perforation | 0 | 11 (100%) | 0 |
| Meckel’s intussusception | 0 | 0 | 2 (22.23%) |
| PVID | 12 (60%) | 0 | 0 |
| Umbilical cyst | 2 (10%) | 0 | 0 |
| Umbilical polyp | 6 (30%) | 0 | 0 |
| Total (n=40) | 20 (50%) | 11 (27.5%) | 9 (22.5%) |
The authors have tried to segregate the patients into two groups based on age at presentation (during infancy and post-infancy) and compare the clinical presentation between the two groups. The authors also tried to look for any sex predilection for any particular clinical presentation of symptomatic OMD anomalies (Acute abdomen, Intestinal Obstruction, Umbilical Abnormalities) but couldn’t find any. The incidence of Meckel’s diverticulum in present study was 50% (20/40).
The surgical options included wedge resection (n=27 of 40; 67.5%), segmental resection of the bowel with end-to-end anastomosis (n=6 of 40; 15%) and local excision in the region of umbilicus (n=7 of 40; 17.5%) [Table/Fig-6]. Twenty-seven patients managed with wedge excision included 10 patients with PVID, one patient with umbilical polyp, seven patients with Meckel’s diverticulum with an obstructing band and nine patients with perforated Meckel’s diverticulum. Segmental resection was performed for two patients each with intussusception (wherein the Meckel’s diverticulum was the pathological lead point), perforated Meckel’s diverticulum and PVID. Local excision in the region of the umbilicus without breaching the virginity of the abdominal domain was affected in five patients of umbilical polyp and two patients of infected umbilical cyst.
Clinical presentations with operative procedures.
| Clinical presentations | Operative procedures |
|---|
| Wedge resection | Segmental resection | Local excision |
|---|
| Acute abdomen |
| PMD | 9 | 2 | 0 |
| Intestinal obstruction |
| MD with obstructive band | 7 | 0 | 0 |
| Meckel’s intussusception | 0 | 2 | 0 |
| Umbilical abnormalities |
| PVID | 10 | 2 | 0 |
| Umbilical polyp | 1 | 0 | 5 |
| Umbilical cyst | 0 | 0 | 2 |
| Total (n=40) | 27/40 (67.5%) | 6/40 (15%) | 7/40 (17.5%) |
PMD: Perforated Meckel’s diverticulum; MD: Meckel’s Diverticulum; PVID: Patent vitellointestinal duct
Upon comparing the clinical presentation with the surgical treatment offered, it was noted that out of 11 patients with acute abdomen, nine patients underwent wedge resection and two patients underwent segmental resection. Out of nine cases of intestinal obstruction, seven underwent wedge resection and two underwent segmental resection. Out of 20 patients of umbilical abnormalities, 11 patients underwent wedge resection, two patients underwent segmental resection and seven patients underwent local excision.
Length of hospital stay ranged from 4-12 days with mean of 7.33 days. Total of six children developed postoperative complications which included two children who underwent wedge resection, one child who underwent segmental resection and three children who underwent local excision. Out of these six children, 5 (12.25%) developed surgical site infections and one child had leak from the anastomosis site which was managed by revising the anastomosis [Table/Fig-7]. On comparing the complications with the surgical procedure performed using Fisher’s-exact test, data was not statistically significant [Table/Fig-8].
Postoperative complications.
| Complications | n (%) |
|---|
| Surgical site infection | 5/40 (12.5%) |
| Anastomotic leak | 1/40 (2.5%) |
| Total | 6/40 (15%) |
Complications in relation to surgical procedure performed.
| Surgical procedure | Complications | No Complications | Total | p-value |
|---|
| Wedge resection | 2 | 25 | 27 | 0.0749 |
| Segmental resection | 1 | 5 | 6 | 1 |
| Local excision | 3 | 4 | 7 | 0.0547 |
p-value calculated by using Fisher’s-exact test
p-value <0.05 is statistically significant
On HPE, ectopic gastric mucosa [Table/Fig-9] was identified in 12 out of 40 patients (30%). Of these 12 patients, six patients (50%) had perforated Meckel’s diverticulum (Acute abdomen), two patients had intussusception with Meckel’s diverticulum acting as the pathological lead point (Intestinal Obstruction), two patients had umbilical polyp and two patients had PVID (Umbilical abnormality) [Table/Fig-10].
Histopathology film 100x Intestinal Mucosa with ectopic gastric mucosa Haematoxylin & Eosin staining.

Clinical presentations and ectopic gastric tissue.
| Clinical presentation | Ectopic gastric tissue | Percentage |
|---|
| Acute abdomen | 6 | 50 |
| Intestinal obstruction | 2 | 16.67 |
| Umbilical abnormalities | 4 | 33.33 |
| Total | 12 | 100 |
Discussion
The Department of Paediatric Surgery at the parent University conducted 644 surgical procedures under general anaesthesia during the study period. Records revealed that out of these 644 surgical procedure, 40 were of symptomatic OMD anomalies which accounted for approximately 6.21%. Authors could not find any similar data in literature for comparison. As majority of OMD anomalies remain asymptomatic throughout life [7,8], exact prevalence of OMD anomalies cannot be predicted. In addition, these patients are operated by general and paediatric surgeons alike; therefore, true prevalence of such anomalies is nothing more than a far-fetched imagination [11].
According to Meckel JF, the frequency of complications due to Meckel’s diverticulum was 25%, but it ranges from 4-16% in recent literature [12,13]. Its incidence in males and females was found to be equivalent however, occurrence of complications can be three to four times more common in males [12]. In the present study also, males were 2.6 times more commonly affected than females reinforcing Meckel’s JF observation that symptomatic OMD anomalies are more common in males and is also in agreement with the other studies reported [7,8,12-14]. In the present study, the age of the cohort ranged from 2 months to 131 months with the mean of 41.77 months and median of 24 months which is comparable with the study of Durakbasa CU et al., who reported age range from 1 day to 15 years with median of 36 months [14]. All the cases in the present study were sporadic with no other congenital anomaly which agrees with the reported results of study done by Durakbasa CU et al., [14].
Vane DW et al., found that 80% of the children who presented with symptomatic OMD anomalies were less than two years of age [2]. In the present study only 21 patients out of 40 were below two years of age constituting 52.5% which is less than the value reported by Vane DW et al., [2]. Possible explanation for this can be the study population. The present study has been done in Asians and in rural areas where parents were not quite aware of these anomalies and ignored them until they complicated. This fact can also be revisited in light that India is a third world country where a large population is struggling to make a daily living [15]. Problems as minor as the OMD anomalies do not figure out on the priority list of the parents who have to spare a day’s wage to visit a health facility [15]. Yet another fact which merits mention here is that the poor will mostly visit a government funded health-facility. All government hospitals in India will cater to routine (non-emergency) problems in their OPDs which run during the working hours; the poor have to choose between a daily wage and a hospital visit.
In the present study, the commonest clinical presentation of the symptomatic OMD anomalies was umbilical abnormalities in 50% followed by acute abdomen in 27.5% and intestinal obstruction in 22.5%, which is contrary to the other studies of Durakbasa CU et al., and Park JJ et al., who reported intestinal obstruction as the commonest clinical presentation of symptomatic OMD anomalies in children (35.5% reported by Durakbasa CU et al., and 40% reported by Park JJ et al.,) [14,16]. Also, on comparing the clinical presentations in infancy and post-infancy group in the present study, it was observed that incidence of umbilical abnormalities was significantly higher in infancy (p=0.0039*). The higher incidence of umbilical abnormalities in infancy can be possibly due to the fact that visible abnormalities like umbilical discharge, PVID, umbilical polyp and umbilical cyst are more likely to be observed by parents earlier than the clinical presentations of intestinal obstruction and acute abdomen which present later as complications of OMD anomalies. Although, intussusception as a result of OMD remnants is a well-known cause of intestinal obstruction, obstruction mainly occurred by way of mesodiverticular bands in the present study. The incidence of Meckel’s diverticulum in the present study was 50% (20/40). This can be attributed to the fact that the incidence of Meckel’s diverticulum in the present study is actually of its complications and not Meckel’s diverticulum per se as asymptomatic Meckel’s diverticulum may remain undiagnosed throughout life [7,8,17]. Many of the patients of Meckel’s diverticulum are asymptomatic [17] and are incidentally diagnosed at laparotomy for other causes and this group of patients were not included in the study. Rectal bleeding is another mode of presentation of symptomatic OMD anomaly and has been reported in other studies as well [7,14,16,18,19]. Several authors reported bleeding as the most common mode of presentation in children [7,16]. In the present study, no case of OMD anomaly presenting with rectal bleed was observed. This can be attributed to the fact that this University lacks a trained radiologist and nuclear imaging facility which is essential for diagnosing OMD anomaly like Meckel’s diverticulum presenting as rectal bleed [20,21]. Also, the patients who presented with rectal bleed and were suspected of having symptomatic OMD anomalies after ruling out other causes and who were referred to higher centres for nuclear imaging may not have returned to us for follow-up.
In the present study, wedge resection was the commonest surgical procedure performed in 67.5% of cases. This is in agreement with the other study of Durakbasa CU et al., in which 32 out of 59 (54.23%) surgical procedures were wedge resections [14]. In the present study, the incidence of ectopic tissue in the OMD remnants is 30% (12/40) which is in agreement with the study done by Durakbasa CU et al., [14]. All these ectopic tissues were of gastric origin and no pancreatic tissue was noted in the present study. The presence of ectopic tissues in the remnants of OMD is a well-known fact. The frequency of finding ectopic tissue is higher in symptomatic cases of OMD remnants [7,16]. In one study, the incidence of ectopic tissue was 59% in symptomatic Meckel’s diverticulum patients of paediatric age group [16]. The most commonly encountered ectopic tissue in OMD remnants is of either gastric or pancreatic origin [16]. Out of 12 patients having ectopic gastric tissue in their OMD remnants specimen six patients (50%) presented with acute abdomen as perforated Meckel’s diverticulum which is in agreement with the study by Park JJ et al., [16].
Other than the obvious umbilical abnormalities, prompt preoperative diagnosis of other OMD remnants is uncommon and difficult. Surgical excision is the treatment of choice for symptomatic OMD remnants either laparoscopically or by open technique [22]. As it is controversial that simple palpation of the diverticulum can help in detecting the ectopic tissue and wedge resection harbours the risk of leaving behind the ectopic tissue many surgeons prefer segmental resection [23]. It is also unknown whether the ectopic tissue left behind ever becomes symptomatic [16].
Limitation(s)
There were certain limitations of the study which included a small sample size and retrospective nature of the study. Further more, the absence of a qualified radiologist and nuclear imaging facility at the University, which is essential for the diagnosis of OMD abnormalities presenting as rectal bleed, one of the clinical presentations of symptomatic OMD anomalies, has resulted in exclusion of such patients from the study.
Conclusion(s)
Symptomatic OMD anomalies were more common in males and presented as acute abdomen, intestinal obstruction, umbilical abnormalities and sometimes with per rectal bleeding. Umbilical abnormalities were the most common presentation of symptomatic OMD anomalies in the present study. Patients presenting with umbilical abnormalities were more common in infancy. Surgery is the treatment of choice in symptomatic OMD remnants. Presence of ectopic tissue is commoner in patients with symptomatic OMD remnants.
UA: Umbilical Abnormalities; AA: Acute Abdomen; IO: Intestinal Obstruction
p<0.05* statistically significant (Chi-square test)
PMD: Perforated Meckel’s diverticulum; MD: Meckel’s Diverticulum; PVID: Patent vitellointestinal duct
p-value calculated by using Fisher’s-exact test
p-value <0.05 is statistically significant