Subarachnoid block is the most commonly used regional anaesthesia technique for lower abdominal surgeries. Local anaesthetics provide analgesia and anaesthesia for various surgical and non surgical procedures. They produce reversible conduction blockade of central and peripheral transmission of autonomic, somatic sensory and motor impulses producing sensory anaesthesia and motor blockade in the innervated area.
Commonly used anaesthetic agents are bupivacaine, ropivacaine and levobupivacaine. They have longer duration of action and are associated with delayed hospital discharge [1]. The ideal anaesthetic for spinal anaesthesia in brachytherapy procedure patients should provide rapid onset of action, adequate potency and predictable (short) duration. Low doses of Bupivacaine, Ropivacaine and Levobupivacaine are associated with longer hospital stay and are less reliable interms of efficacy, onset and spread [2].
In comparison with bupivacaine, chloroprocaine had early recovery from anaesthesia, early mobilisation and faster recovery from hospital. This infers that low dose of chloroprocaine is an alternative to low doses of long-acting local anaesthetics in short duration day care procedures [3,4].
Chloroprocaine, like that of lidocaine has short latency and short duration. A 2-chloroprocaine is an amino-ester local anaesthetic with a very short half-life. The drug was synthesised by Rubin, Marks, Wishinsky and Lanzilotti in 1946, and has been advocated only for local infiltration, regional block and epidural anaesthesia [5]. It has been successfully used for spinal anaesthesia since 1952 [6]. Neurotoxicity has been associated with large doses of 2-chloroprocaine as an epidural anesthesia, leading to its withdrawal commercially [7].
The combination of low pH (<3) and the presence of sodium bisulfite, an antioxidant, may have been responsible for the neurotoxicity [8-10]. Subsequently, the pH of the solution has been adjusted and a preservative free formulation was reintroduced into clinical use in 2005 [11]. This new formulation has been safely used for spinal anaesthesia in healthy volunteers and in patients without complications [12-16].
The aim of this study was to assess the time of onset, duration of anaesthesia, two segment regression, complete regression of spinal anaesthesia, and secondary outcome was to measure haemodynamic parameters like Heart Rate (HR), systolic and diastolic blood pressure and Mean Arterial Pressure (MAP) in 20 mg chloroprocaine and 30 mg chloroprocaine groups.
Materials and Methods
A prospective randomised clinical study was conducted in 140 female patients of 18-60 years of age, undergoing elective brachytherapy procedure for carcinoma cervix were enrolled for the study. After approval (KMIO/MEC/020/05 JAN2018) by the Institutional Ethics Committee the study was conducted in the Department of Anaesthesia and Pain Relief, Kidwai Memorial Institute of Oncology, Bangalore. The study period was from September 2017 to April 2019. The study was registered with Central Trial Registry-India with the registration number CTRI/2019/06/019567.
Preanaesthetic evaluation of patients satisfying the inclusion criteria was carried out and informed written consent was obtained. The study subjects were randomly allotted into two groups by a computer-generated random number table [Table/Fig-1].
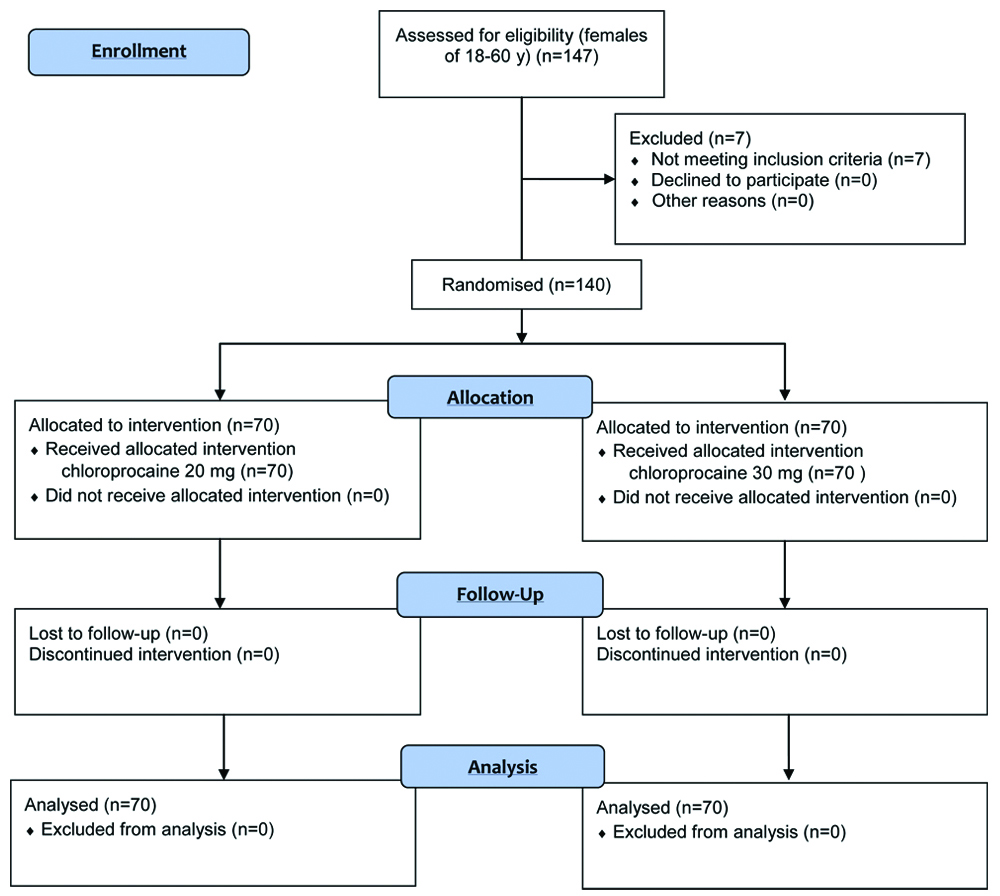
Patients aged between 18 to 60 years, belonging to ASA physical status Grade I and Grade II and consenting for study were included. Exclusion criteria were patients who refused to give informed consent for study, ASA physical status III and IV, and patients contraindicated for neuraxial blockade.
Sample size was calculated based on 95% confidence interval with 90% power of study by using the formula.
N=(Z α/2+Zβ)*2*σ2/d2
{Here, Z α/2 is the critical value of normal distribution at α/2 (e.g., for a confidence level of 95%, α is 0.05 and the critical value is 1.96), Z, is the critical value of the Normal Distribution at β (e.g., or a power of 80%, β is 0.2 and the critical value is 0.84), σ2 is the population variance and difference we would like to detect, sample size is 70 in each group.}.
Procedure
Patients were randomised using computer generated table and assigned to one of the two groups:
Group A: Chloroprocaine 20 mg: 70 patients.
Group B: Chloroprocaine 30 mg: 70 patients.
A routine preanaesthetic examination was conducted prior to brachytherapy, assessing patients general condition and optimising the patients before taking for procedure. The patients were premedicated with tablet alprazolam 0.5 mg and tablet pantoprazole 40 mg orally at bed time on the previous night before procedure. They were kept nil orally for six hours prior to procedure. On the day of procedure, Preanaesthetic Checkup (PAC) was reviewed. ASA Standard monitors were attached and base line readings noted. Intravenous (i.v.) line was obtained with 18 gauge cannula. Under aseptic precautions lumbar puncture was done using 25G spinal needle at L2-L3/L3-L4 inter space with the patients in the right or left lateral decubitus position. Under aseptic precautions the study drug chloroprocaine was loaded in a 5 mL syringe by other anaesthesiologist (BLINDING) who was not involved in the study. Just before spinal anaesthesia, syringe was handed over to the anaesthesiologist performing the subarachnoid block. Patients in Group A received Inj, Chloroprocaine 1% 2 mL (20 mg) intrathecally and Group B patients received Inj. Chloroprocaine 1% 3 mL (30 mg) intrathecally. Patients were made to lie down in the supine posture immediately after the subarachnoid injection of the study drug, keeping the table neutral position. Patient’s haemodynamic parameters were monitored at 3 minutes interval for first 10 minutes, then every 5 minutes for 60 minutes or till the end of the surgery. Patients were observed for the next 24 hours at 4,8,12,24 hours postoperatively.
The following parameters were observed:
1) HR, non invasive blood pressure (SBP, DBP and MAP).
2) Time for onset of sensory blockade was assessed by loss of pain sensation bilaterally along midclavicular line to pin prick till T10 dermatomal level (23G Hypodermic needle).
3) Time for onset of motor blockade was noted (modified Bromage score 0).
4) Time for two segment regression (time taken for the sensory level to regress by two segments in minutes).
5) Total duration of sensory blockade and total duration of motor blockade (time to regress to level L1 from the peak block).
6) Any intraoperative and postoperative side-effects like shivering, nausea and vomiting were observed.
Statistical Analysis
SPSS 15.0 was used for statistical analysis. Continuous measurements are presented on Mean±SD and results on categorical measurements are presented in number (%). Significance was assessed at 5% level of significance.
Student t-test (two-tailed, independent) has been used to find the significance of study parameters on continuous scale between two groups (Intergroup analysis) on metric parameters.
Chi-square/Fisher-Exact test has been used to find the significance of study parameters on categorical scale between two or more groups.
Results
Patients in both the groups had no significant differences in terms of Age, ASA physical status and anthropometric variables [Table/Fig-2].
Summary of Demographic characteristics.
| Parameter | Group | Mean | Std. dev | SE of mean | Mean difference | p-value |
|---|
| Age (years) | Group A | 51.18 | 9.83 | 1.17 | 2.483 | 0.095 |
| Group B | 48.70 | 7.56 | 0.90 |
| Weight (Kg) | Group A | 59.20 | 5.68 | 0.67 | 0.683 | 0.495 |
| Group B | 58.51 | 6.16 | 0.74 |
| Height (cm) | Group A | 159.86 | 5.22 | 0.62 | 1.131 | 0.186 |
| Group B | 158.73 | 4.86 | 0.58 |
SE: Standard error
The time of onset of sensory and motor block, time for two segment regression and total duration of analgesia in both the groups is shown in [Table/Fig-3]. The maximum sensory level achieved was T8 in Group A, while T6 in group B, respectively.
Comparison of study variables among groups.
| Parameter | Group | Mean | Std. dev | SE of mean | Mean difference | p-value |
|---|
| Time of onset for sensory block (min) | Group A | 4.32 | 1.35 | 0.16 | 0.181 | 0.417 |
| Group B | 4.14 | 1.29 | 0.15 |
| Time of onset for motor block (min) | Group A | 5.45 | 1.51 | 0.18 | 0.922 | <0.001* |
| Group B | 4.53 | 1.05 | 0.12 |
| Time for two segment regression (min) | Group A | 23.73 | 4.83 | 0.57 | 1.447 | 0.068 |
| Group B | 22.29 | 4.48 | 0.54 |
| Total duration of sensory block (min) | Group A | 48.66 | 8.90 | 1.06 | -1.838 | 0.224 |
| Group B | 50.50 | 8.97 | 1.07 |
| Total duration of motor block (min) | Group A | 69.44 | 12.86 | 1.53 | -18.278 | <0.001* |
| Group B | 87.71 | 7.05 | 0.84 |
| Total duration of analgesia (min) | Group A | 50.49 | 7.28 | 0.86 | -12.578 | <0.001* |
| Group B | 63.07 | 14.28 | 1.71 |
* p-value<0.05 was considered significant
There was no statistically significant difference found in terms of haemodynamic variables like HR [Table/Fig-4] among both groups during perioperative period.
Comparison of Heart rate (HR) among both groups.
| Time interval | Group | Mean | Std. dev | p-value |
|---|
| Baseline | Group A | 96.48 | 21.65 | 0.619 |
| Group B | 94.53 | 24.68 |
| 0 min | Group A | 93.65 | 20.61 | 0.972 |
| Group B | 93.51 | 24.24 |
| 3 min | Group A | 95.03 | 21.95 | 0.709 |
| Group B | 93.56 | 24.66 |
| 6 min | Group A | 91.82 | 19.18 | 0.760 |
| Group B | 92.91 | 23.25 |
| 9 min | Group A | 94.54 | 21.06 | 0.662 |
| Group B | 92.87 | 24.02 |
| 12 min | Group A | 90.27 | 20.89 | 0.846 |
| Group B | 90.99 | 22.91 |
| 15 min | Group A | 94.04 | 20.27 | 0.494 |
| Group B | 91.57 | 22.48 |
| 20 min | Group A | 92.68 | 22.11 | 0.500 |
| Group B | 90.07 | 23.66 |
| 25 min | Group A | 91.96 | 23.81 | 0.376 |
| Group B | 88.39 | 23.99 |
| 30 min | Group A | 91.56 | 22.44 | 0.397 |
| Group B | 88.24 | 23.96 |
| 40 min | Group A | 90.97 | 23.29 | 0.427 |
| Group B | 87.76 | 24.60 |
| 50 min | Group A | 91.72 | 22.20 | 0.350 |
| Group B | 88.11 | 23.41 |
| 60 min | Group A | 92.41 | 23.78 | 0.321 |
| Group B | 88.31 | 25.07 |
| 70 min | Group A | 90.21 | 23.75 | 0.553 |
| Group B | 87.76 | 25.29 |
| 4 hours | Group A | 89.44 | 23.65 | 0.487 |
| Group B | 86.57 | 25.20 |
| 8 hours | Group A | 84.41 | 24.30 | 0.909 |
| Group B | 83.93 | 25.58 |
| 12 hours | Group A | 86.48 | 22.86 | 0.815 |
| Group B | 85.54 | 24.65 |
| 24 hours | Group A | 82.61 | 24.48 | 0.993 |
| Group B | 82.64 | 25.55 |
The SBP was comparable at the sixth minute between two groups and it was statistically significant (p<0.05), while rest of the duration the SBP values were non significant [Table/Fig-5].
Comparison of Systolic Blood Pressure (SBP) among both groups.
| Time Interval | Group | Mean (mmHg) | Std. dev | p-value |
|---|
| Baseline | Group A | 137.25 | 11.98 | 0.994 |
| Group B | 137.27 | 15.17 |
| 0 min | Group A | 135.37 | 10.52 | 0.907 |
| Group B | 135.61 | 14.34 |
| 3 min | Group A | 124.13 | 16.05 | 0.740 |
| Group B | 123.17 | 17.98 |
| 6 min | Group A | 116.25 | 19.00 | 0.023* |
| Group B | 123.39 | 17.71 |
| 9 min | Group A | 131.20 | 12.94 | 0.213 |
| Group B | 128.30 | 14.51 |
| 12 min | Group A | 118.87 | 11.53 | 0.310 |
| Group B | 121.16 | 14.83 |
| 15 min | Group A | 118.15 | 9.86 | 0.769 |
| Group B | 117.60 | 12.40 |
| 20 min | Group A | 118.20 | 10.85 | 0.743 |
| Group B | 117.56 | 12.21 |
| 25 min | Group A | 118.35 | 10.81 | 0.898 |
| Group B | 118.10 | 12.49 |
| 30 min | Group A | 116.51 | 11.77 | 0.921 |
| Group B | 116.71 | 12.97 |
| 40 min | Group A | 117.92 | 11.03 | 0.650 |
| Group B | 116.99 | 13.16 |
| 50 min | Group A | 116.01 | 11.23 | 0.905 |
| Group B | 115.77 | 12.73 |
| 60 min | Group A | 117.10 | 10.19 | 0.780 |
| Group B | 116.56 | 12.60 |
| 70 min | Group A | 114.51 | 12.28 | 0.946 |
| Group B | 114.66 | 13.85 |
| 4 hours | Group A | 114.51 | 12.28 | 0.961 |
| Group B | 114.61 | 13.82 |
| 8 hours | Group A | 114.51 | 12.28 | 0.966 |
| Group B | 114.60 | 13.81 |
| 12 hours | Group A | 114.51 | 12.28 | 0.951 |
| Group B | 114.64 | 13.84 |
| 24 hours | Group A | 114.51 | 12.28 | 0.961 |
| Group B | 114.61 | 13.82 |
* p-value<0.05 was considered significant
There was statistical difference found for diastolic blood pressure among two groups at ninth minute with p<0.05. During rest of the procedure the values were non significant. [Table/Fig-6].
Comparison of Diastolic Blood Pressure (DBP) among both groups.
| Time Interval | Group | Mean | Std. dev | p-value |
|---|
| Baseline | Group A | 84.46 | 6.17 | 0.803 |
| Group B | 84.81 | 9.96 |
| 0 min | Group A | 81.01 | 3.03 | 0.776 |
| Group B | 81.30 | 7.81 |
| 3 min | Group A | 77.45 | 8.88 | 0.683 |
| Group B | 78.10 | 9.96 |
| 6 min | Group A | 73.68 | 12.42 | 0.239 |
| Group B | 75.93 | 10.07 |
| 9 min | Group A | 81.99 | 10.77 | 0.004* |
| Group B | 76.71 | 10.80 |
| 12 min | Group A | 74.03 | 6.64 | 0.547 |
| Group B | 73.17 | 9.88 |
| 15 min | Group A | 74.55 | 6.62 | 0.854 |
| Group B | 74.79 | 8.47 |
| 20 min | Group A | 74.58 | 8.12 | 0.718 |
| Group B | 74.10 | 7.51 |
| 25 min | Group A | 71.45 | 6.34 | 0.459 |
| Group B | 70.60 | 7.23 |
| 30 min | Group A | 71.04 | 6.09 | 0.171 |
| Group B | 69.53 | 6.93 |
| 40 min | Group A | 68.93 | 7.95 | 0.965 |
| Group B | 68.99 | 7.30 |
| 50 min | Group A | 68.93 | 7.95 | 0.990 |
| Group B | 68.91 | 7.16 |
| 60 min | Group A | 67.32 | 8.94 | 0.785 |
| Group B | 67.71 | 8.02 |
| 70 min | Group A | 67.32 | 8.94 | 0.785 |
| Group B | 67.71 | 8.02 |
| 4 hours | Group A | 67.32 | 8.94 | 0.785 |
| Group B | 67.71 | 8.02 |
| 8 hours | Group A | 67.32 | 8.94 | 0.785 |
| Group B | 67.71 | 8.02 |
| 12 hours | Group A | 67.32 | 8.94 | 0.785 |
| Group B | 67.71 | 8.02 |
| 24 hours | Group A | 67.32 | 8.94 | 0.785 |
| Group B | 67.71 | 8.02 |
* p-value<0.05 was considered significant
The MAP measured did not show any clinical and statistical significance during brachytherapy procedure [Table/Fig-7].
Comparison of Mean Arterial Pressure among both groups (MAP).
| Time interval | Group | n | Mean | Std. dev | SE of mean | Mean difference | p-value |
|---|
| Baseline | Group A | 70 | 101.83 | 13.70 | 1.63 | 0.702 | 0.766 |
| Group B | 70 | 101.13 | 14.28 | 1.71 |
| 0 min | Group A | 70 | 100.96 | 5.17 | 0.61 | 0.001 | 1.000 |
| Group B | 70 | 100.96 | 8.74 | 1.04 |
| 3 min | Group A | 70 | 94.23 | 12.83 | 1.52 | 0.940 | 0.680 |
| Group B | 70 | 93.29 | 14.12 | 1.69 |
| 6 min | Group A | 70 | 88.54 | 13.38 | 1.59 | -2.551 | 0.229 |
| Group B | 70 | 91.09 | 11.59 | 1.39 |
| 9 min | Group A | 70 | 94.96 | 11.58 | 1.37 | 3.358 | 0.089 |
| Group B | 70 | 91.60 | 11.73 | 1.40 |
| 12 min | Group A | 70 | 89.39 | 6.65 | 0.79 | -0.363 | 0.781 |
| Group B | 70 | 89.76 | 8.68 | 1.04 |
| 15 min | Group A | 70 | 88.80 | 7.81 | 0.93 | -0.154 | 0.910 |
| Group B | 70 | 88.96 | 8.39 | 1.00 |
| 20 min | Group A | 70 | 88.39 | 8.83 | 1.05 | -0.232 | 0.870 |
| Group B | 67 | 88.63 | 7.82 | 0.95 |
| 25 min | Group A | 70 | 89.07 | 7.66 | 0.91 | 0.499 | 0.740 |
| Group B | 63 | 88.57 | 9.67 | 1.22 |
| 30 min | Group A | 70 | 87.97 | 6.92 | 0.82 | 0.572 | 0.661 |
| Group B | 60 | 87.40 | 7.95 | 1.03 |
[Table/Fig-8] infers that there was no significant difference in terms of use of Inj. mephentermine among both the groups.
Use of Mephentermine among both groups.
| Mephentermine | Group A | Group B | χ2 | p-value |
|---|
| n | % | n | % |
|---|
| Used | 8 | 11% | 9 | 13% | 0.067 | 0.796 |
| Not used | 62 | 89% | 61 | 87% |
| Total | 70 | 100% | 70 | 100% |
These patients that had electrodes of brachytherapy postprocedure were routinely receiving injection tramadol and injection paracetamol for analgesia. Patients were observed for any hypersensitivity reactions for the drug, nausea, vomiting and shivering during perioperative period and none of them had side-effects in either of two groups.
Discussion
Brachytherapy is a short duration procedure wherein adequate sensory block would suffice more than motor block. After the release of preservative free chloroprocaine it has become an alternate drug for the subarachnoid block for short duration procedures. The purpose of the study was to compare two doses of chloroprocaine for spinal anaesthesia for brachytherapy procedures. Our principal finding was that spinal anaesthesia with 30 mg chloroprocaine can provide satisfactory surgical block while permitting earlier discharge from hospital without any side-effects. According to the experimental results mentioned above, the same concentration of two different doses of chloroprocaine has different anaesthetic effect in brachytherapy procedure. The patients in two groups were able to actively cooperate when changing position or transporting in intraoperative, postoperative process. Blood pressure, HR, oxygen saturation remained stable.
The onset of sensory blockade was earlier with group B when compared to group A, but the difference was statistically not significant. Among various doses of 2-chloroprocaine to compare the onset of sensory blockade the reason for the fast onset was attributed to difference in volumes of two drugs. Kopacz DJ, established that the minimum effective dose to lower limit of hyperbaricity as 1.00100 g/mL based on previous measurements of the density of human Cerebrospinal Fluid (CSF). They also measured the density of plain preservative-free 2-chloroprocaine as 1.00123 g/mL and found as marginally hyperbaric when compared to CSF [16]. Warren DT and Kopacz DJ, demonstrated that adding a small amount (0.8-1.1%) of dextrose to spinal local anaesthetic increases their baricity and produces the benefits of a faster onset of block and a reduction in the variability of peak block level. When compared to other local anaesthetics like bupivacaine, ropivacaine and lignocaine the baricity of chloroprocaine was less and this marginally less hyperbaricity can be accounted for the delayed onset of action with chloroprocaine [17].
The maximum sensory level achieved was higher in group B when compared to group A. The difference in levels of blockade may be linked to factors like different volumes of drugs, dose, baricity, site of injection, direction of needle and also barbotage technique. It is also depends on the anthropometric characteristics of the patients. Increase in dose (milligrams), especially with plain solutions, causes higher spread and longer duration of anaesthesia.
Kopacz DJ, used intrathecal chloroprocaine in healthy volunteers to establish the minimum effective dose observed that (chloroprocaine 20 mg) produced a level of sensory anaesthesia of at least L1 in all subjects and maximum level of blockade to be at T9 in 20 mg chloroprocaine and and T8 in 30 mg chloroprocaine group [16]. They also found that with increasing dose there was an increase in level of blockade and also duration of block. Smith KN et al., compared 30 mg, 45 mg and 60 mg of intrathecal chloroprocaine in healthy volunteers and observed the peak block height reached was T5 (range, C5-L3) and which correlated positively with increasing dose. With 30, 45, and 60 mg, peak block heights and ranges were as follows: T7, T5, and T2. Anaesthetic substances with a higher density than CSF are hyperbaric while those with lower density are hypobaric. The results of this study were attributed to the 3% hyperbaric chloroprocaine solution [18].
The mean two regression time in group A was 23.73 minutes and 22.29 minutes in group B which was clinically of not much clinical difference and it was statically insignificant. Kopacz DJ observed that two segment regression with 20 mg chloroprocaine was 37 minutes, with 30 mg was 51 minutes, with 40 mg it was 45 minutes and with chloroprocaine 60 mg was 43 minutes, respectively. The varied results in different studies were due to different volume and doses (1% vs 2%) that may have accounted for the change in time for two segment regression [16].
The total duration of sensory block was 48.66 minutes in group A and 50.50 minutes in group B which was statistically insignificant. Kopacz DJ demonstrated that the time taken to regress to the level of L1 in chloroprocaine 20 mg was 40 minutes and chloroprocaine 30 mg was 42 minutes. But in our study we found that the duration was little more compared clinically [16].
The time to attain motor block of Bromage grade 3 (onset time) from intrathecal injection was 5.44+1.47 minutes in group A and 4.54+1.03 minutes in group B (p-value <0.001). When compared to other local anaesthetics like bupivacaine the baricity of chloroprocaine is less and this can be accounted for the delayed onset of action of motor blockade but there are no studies to substantiate these actions (chloroprocaine with bupivacaine).
According to the experimental results of Zhang Y et al., the same concentration of different doses of chloroprocaine has different anaesthetic effect in surgery of saddle anaesthesia. The authors used 0.5% (w/v) chloroprocaine dissolved in 0.6-1.0 mL 10% (w/v) glucose solution. The patients were able to actively cooperate when changing position or transporting in intraoperative, postoperative process and the variables like blood pressure, HR, oxygen saturation remained stable [19].
The total duration of motor blockade that is time to reach a bromage scale 1 from intrathecal injection was 69 minutes in group A and 87 minutes in group B and this difference was statistically significant (p-value <0.001). Breebart MB et al., observed in 100 patients the onset and quality of the block between chloroprocaine (40 mg) and lignocaine (60 mg). They observed that onset and quality of the block were comparable between two groups. Time to regain bromage 1 and L2 regression were shorter for the chloroprocaine group compared with the lignocaine group. Voiding and discharge were approximately 40 minutes faster for the chloroprocaine group compared with the lignocaine group. They also observed that chloroprocaine group was discharged faster than lignocaine group. They concluded that chloroprocaine is suitable for day-care surgery because of faster block regression and discharge than lidocaine [20].
Kopacz DJ in their dose ranging study (10-60 mg), found that 10 mg of 2-chloroprocaine has no effect, whereas 20 mg and 30 mg produced sensory anaesthesia adequate for surgical procedures but with less motor block and some cases of sacral sparing should be anticipated [16]. These results are in accordance with this study wherein 30 mg of drug was more useful for brachytherapy procedures.
The study differed in terms of onset of action for sensory and motor block, two segment regression time and total duration of block from previous studies attributing to varying doses and concentration of chloroprocaine [16-18,20]. None of the patients in either groups had side-effects like shivering, nausea and vomiting.
Limitation(s)
Overall, the study limitations were comparison of two different volumes of drug which would have resulted in differences in characteristics of spinal block. Secondly patients for brachytherapy with tandems in place for 24 hours needed more analgesia and had decreased patient satisfaction, when compared to patients with other long acting local anaesthetics.
Conclusion(s)
In conclusion, 30 mg spinal 2-chloroprocaine provides adequate duration and density of spinal anaesthesia for brachytherapy procedures as compared with 20 mg spinal 2-chloroprocaine with no side-effects.
SE: Standard error
* p-value<0.05 was considered significant
* p-value<0.05 was considered significant
* p-value<0.05 was considered significant