Comparative Evaluation of Modified Endodontic Sealers’ Penetration Following Sonic Activation of Irrigants: A Scanning Electron Microscope Study
Vincia Valencio D’Souza1, Rushikesh Ramesh Mahaparale2, Tiptur Manjunath Mangala3, Adish Anand Saraf4, Sneha Ramling Mali5, Sagar Pawar6, Shubham Anil Mandhane7, Shraddha Rajendra Nahar8
1 Postgraduate Student, Department of Conservative Dentistry and Endodontics, School of Dental Sciences, KIMSDU, Karad, Maharashtra, India.
2 Reader, Department of Conservative Dentistry and Endodontics, School of Dental Sciences, KIMSDU, Karad, Maharashtra, India.
3 Professor and Head, Department of Conservative Dentistry and Endodontics, School of Dental Sciences, KIMSDU, Karad, Maharashtra, India.
4 Senior Lecturer, Department of Conservative Dentistry and Endodontics, School of Dental Sciences, KIMSDU, Karad, Maharashtra, India.
5 Senior Lecturer, Department of Conservative Dentistry and Endodontics, School of Dental Sciences, KIMSDU, Karad, Maharashtra, India.
6 Senior Lecturer, Department of Conservative Dentistry and Endodontics, School of Dental Sciences, KIMSDU, Karad, Maharashtra, India.
7 Postgraduate Student, Department of Conservative Dentistry and Endodontics, School of Dental Sciences, KIMSDU, Karad, Maharashtra, India.
8 Postgraduate Student, Department of Conservative Dentistry and Endodontics, School of Dental Sciences, KIMSDU, Karad, Maharashtra, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Vincia Valencio D’souza, B101, Dhruv Tara Housing Society (DK Tower), Sector 20 D, Plot 18B, Airoli, Navi Mumbai, Maharashtra, India.
E-mail: vincids94@gmail.com
Introduction
When sealer is adapted well to the root canal wall, there is less tendency for microleakage and strength of root canal is enhanced. Therefore, it is important for the sealer to possess good tubular penetration depth and adaptation to dentinal walls.
Aim
To compare the effects of sonic activation of 5.25% Sodium Hypochlorite (NaOCl) and incorporation of Zinc Oxide (ZnO) nanoparticles in AH Plus sealer on tubular sealer penetration.
Materials and Methods
Forty extracted human mandibular premolars with a single root canal were used for this invitro study from October 2019 to January 2020. All teeth were prepared upto F3 with Pro Taper Universal rotary system. Teeth were randomly divided into four groups of 10 teeth each. Group I (control) included teeth obturated using AH plus sealer, Group II: teeth obturated using AH plus sealer incorporated with ZnO nanoparticles, Group III: teeth prepared along with sonic activation of the irrigant followed by obturation using AH plus sealer and Group IV: teeth prepared along with sonic activation of the irrigants followed by obturation with AH plus sealer incorporated with ZnO nanoparticles. Irrigation was achieved with 5.25% sodium hypochlorite. The canals were dried and obturated using respective sealers and single gutta percha cones. After storage at 37°C and 100% humidity, the roots were split into two halves and sealer penetration in the dentinal tubules was examined by using scanning electron microscope. The values obtained were statistically analysed using One-way Analysis of Variance (ANOVA) with significant p-value less than 0.05.
Results
In all the groups, sealer penetrated into the dentinal tubules. There was enhanced tubular sealer penetration (289.57±59.43 μm) after sonic activation of the irrigant (group 3) followed by that on addition of ZnO nanoparticles to the sealers (group 2) (278.21±71.24 μm) and the least was seen in the control group (group 1) (211.5±66.33 μm). The combined effect of both i.e., use of sonic activation of irrigant followed by obturation using modified sealer with gutta percha (group 4) (318.64±88.27 μm) showed greatest tubular sealer penetration depth.
Conclusion
Incorporation of nanoparticles aids in improving sealer penetration which is further enhanced in case of sonic activation during irrigation.
AH plus sealer, Endoactivator, Zinc oxide nanoparticles
Introduction
Treatment of infected teeth aims to reduce the microbial load within the root canal system to allow healing, and to prevent microbial re-entry. In order to prevent reinfection after root canal treatment, complete removal of the infectious materials in the root canal and obtaining a hermetic seal is essential [1]. The remaining bacteria and their products have the tendency to enter the dentinal tubules which may result in the reinfection of root canal system. Thus, to prevent reinfection after root canal treatment it is crucial to remove the infectious materials in the root canal completely and obtain a hermetic seal. Obturation of the complex root canal system should be done using filling materials which can embed the remaining bacteria and prevent the conveyance between root canal and apical tissues owing to its superior sealing ability [2].
Gutta percha has been the most commonly used root canal obturating material but it lacks the ability to adhere to the dentinal wall [3]. This can lead to microleakage and failure of the endodontic treatment. The sealer can fill the irregularities of the root canal wall and the dentinal tubules, which cannot be filled by gutta percha. AH Plus root canal sealer is a resin-based formula with excellent radiopacity, low shrinkage, low solubility and outstanding flow characteristics. It is biocompatible and paste/paste system enables fast, easy mixing and dosage control [4]. Zinc oxide nanoparticles have attracted much attention due to their versatile and promising applications in medical field, being an antibacterial, antifungal, and antifouling agent. It has many advantages such as chemical stability, thermal resistance, robustness, and long shelf life [5].
Good adaptation between the root canal wall and the sealer will augment the strength of the tooth and increase the fracture resistance of the tooth along with reducing the chance of microleakage significantly. Therefore, good adaptation and tubular penetration depth are important properties for an ideal sealer [2]. Thus, the aim of this study was to compare the effects of sonic activation of 5.25% NaOCl and incorporation of ZnO nanoparticles in AH Plus sealer on tubular sealer penetration.
Materials and Methods
This is an invitro study performed in Department of Conservative Dentistry and Endodontics, School of Dental Sciences, KIMSDU, Karad, Maharashtra, India, in the duration from October 2019 to January 2020, after obtaining the Ethical Committee Approval (protocol no is 271/2019-2020).
Selection of Tooth Samples
Inclusion criteria: Forty single-rooted, non-carious human mandibular premolars extracted for orthodontic purpose with similar dimensions and single canal were used for this study.
Exclusion criteria: Radiographical assessment was done to exclude the samples having more than one canal, presence of internal resorption or calcifications.
Standardised root length of 10 mm were obtained for all samples after the teeth were decoronated with a diamond disc (Kerr Dental) under water coolant. The samples were then randomly divided into 4 groups consisting of 10 samples each (n=10) [Table/Fig-1] ZnO nanopowder (SN Chemicals Pvt., Ltd.,) was added 5% by weight in the AH plus sealer paste (Dentsply Malliefer) and was mixed manually based on prior studies [6,7]. Canals were prepared with Protaper Universal (Dentsply) rotary files till F3 keeping the working length 0.5 mm short of the apex. Intermittent irrigation was done using 2 mL 5.25% sodium hypochlorite (Prime Dental) after each file. 17%EDTA (Prime Dental) was used for removal of smear layer. For the samples in group 3 and 5, Endoactivator (Dentsply) was used for activation of sodium hypochlorite. The canals were finally flushed with 5 mL of normal saline. The canals were dried using sterile paper points and master cone radiograph was taken. Obturation was done using single cone gutta percha and AH plus sealer or modified AH plus sealer as per the group. The canal orifices were sealed with sticky wax. Specimens were stored in 100% relative humidity at 37°C for 24 hour to allow complete set of the obturating material [8]. The samples were sectioned longitudinally by placing grooves on the mesial and distal surfaces with a diamond disc and then separating the sections using a chisel and mallet. Teeth were allowed to dry for 24 hours. The samples were then subjected to gold sputtering and Scanning Electron Microscopy (SEM) analysis was conducted at the site 4 mm from the apex. The penetrability was measured using the scale available when the samples were viewed under the Scanning Electron Microscope (LEICA).
Division of samples into groups (N=40).
| Groups | Samples |
|---|
| 1 | Teeth obturated using AH plus sealer (control group) |
| 2 | Teeth obturated using AH plus sealer incorporated with ZnO nanoparticles (S.N. Chemicals Pvt., Ltd.,) |
| 3 | Teeth prepared along with sonic activation of the irrigant followed by obturation using AH plus sealer |
| 4 | Teeth prepared along with sonic activation of the irrigants followed by obturation with AH plus sealer incorporated with ZnO nanoparticles. |
Statistical Analysis
The values obtained were statistically analysed (Social Science Statistics) using One-way Analysis of Variance (ANOVA) and Post-Hoc Tukey’s. The p-value less than 0.05 were considered to be statistically significant.
Results
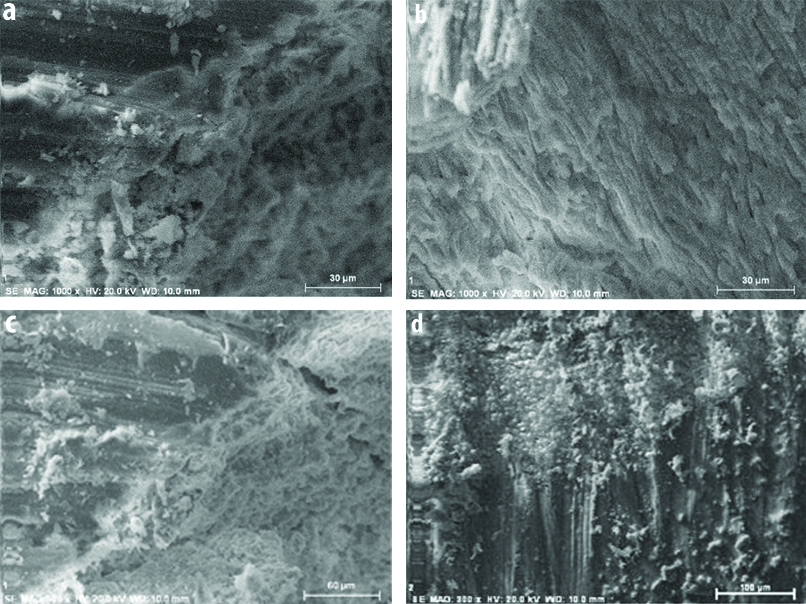
In all the groups, sealer penetrated into the dentinal tubules. Sealer penetration depth was measured in micrometers and result showed that there was minimum tubular sealer penetration depth in the control group (group 1) [Table/Fig-2a]. There was enhanced tubular sealer penetration after sonic activation of the irrigant (group 3) [Table/Fig-2c] followed by that on addition of nanoparticles to the sealers (group 2) [Table/Fig-2b]. There was statistically significant increase in tubular penetration depth in both, group 2 and 3 when compared to the control group. The combined effect of both i.e., use of sonic activation of irrigant followed by obturation using modified sealer with gutta percha (group 4) [Table/Fig-2d] showed greatest tubular sealer penetration depth [Table/Fig-3].
Scanning electron microscope images showing dentinal sealer penetration from the pulp dentin junction: a) High magnification (1000x) SEM image of Group 1 sample (control group; b) SEM image of Group 2 sample; c) SEM image of Group 3 sample; d) SEM image of Group 4 sample.
Result of sealer penetration depth of each group (p<0.05 significant).
| Groups | Sealer penetration depth (μm) | p-value |
|---|
| Group 1 | Group 2 | Group 3 | Group 4 |
|---|
| 1 | 211.5±66.33 | - | - | - | - |
| 2 | 278.21±71.24 | 0.001 | - | - | - |
| 3 | 289.57±59.43 | 0.001 | 0.631 | - | - |
| 4 | 318.64±88.27 | 0.001 | 0.001 | 0.010 | - |
Discussion
Deeper penetration of the endodontic sealer inside the dentinal tubules increases the interface between the core material and dentinal walls, hence leading to mechanical retention of the material via sealer plug interlocking inside the tubules [9]. The improved penetration allows the sealer to better reach the bacteria deeply dislodged inside the dentinal tubules, thus improving its antibacterial effect [10]. The benefits of deep tubule penetration depth is increased contact area between root fillings and dentin, thus, increasing the sealing ability of the root canal system, which hampers the bacteria from getting inside the dentinal tubules and its antimicrobial effects increases in close proximity to the microbes. Also, deep sealer penetration raises the fracture resistance of root canal [2]. Studies have shown that AH plus (resin based sealer) has greater penetration depth than other eugenol based sealers and some resin based sealers as well [11,12].
However, sealer penetration in the dentinal tubules depends on many factors like smear layer removal [13], dentinal permeability (the number and the diameter of tubules), root canal dimension, the physical and chemical properties of the sealer [14,15]. The flow is one of the main factors that influence the tubular penetration and is defined as the ability of a sealer to penetrate in irregularities, lateral canals, or dentinal tubules of the root canal system [16]. The flow of sealer is mainly determined by the consistency and particle size along with other factors such as the internal diameter of the root canal and the rate of insertion [15]. It has been proven that the addition of nanoparticles decreases the viscosity of the endodontic sealer and thus enhances flow of the sealer [17]. Desouky AA et al., showed that the synthesised nano-powder sealers are suitable for use in root canal therapy owing to their improved sealing ability [18].
ZnO has been used in medical applications such as cancer treatment [19,20] and DNA detection [21]. It has also shown good antibacterial properties [22-24] which suggests that it’s powder can be used for dental applications as a sealer [25-27]. ZOE-based cements possess favourable characteristics in terms of biocompatibility. ZnO nanopowders have shown less microleakage in comparison with other root canal sealers, making them suitable for use in Root Canal Treatment [28]. ZnO nanopowders are commercially made available by many companies. It can be easily ordered and since the manipulation does not require any extra equipment, it can be used by clinicians. These were the reasons for selecting ZnO nanoparticles to be incorporated in the sealer in the present study. Incorporation of zinc oxide nanoparticles enhanced the tubular penetration of AH plus sealer which could be attributed to its improved flow owing to the small particle size. Elkateb WM et al., conducted a similar study and concluded that ZnO nanoparticles modified sealer showed improved dentinal tubular penetration of the sealer compared to that of the unmodified control group [29].
Removal of smear layer and proper debridement plays a vital role in disinfection of the root canal system and penetration of the sealer into the dentinal tubules [30]. Thus, it is imperative to maintain good irrigation protocol. Sodium hypochlorite is considered the gold standard for irrigation. It has the capacity to dissolve all the organic materials [31]. In the present study, 5.25% sodium hypochlorite has been used for the irrigation owing to its good antibacterial properties. A 17% EDTA was used for removal of smear layer. Sodium hypochlorite activation has been done in the present study. Many systems are available for activation of irrigants such as sonic and ultrasonic activation systems. In this study, endoactivator i.e., sonic activation has been used owing to its easy availability, safe to use and effective cleansing ability [32]. EndoActivator is a sonically driven irrigation system that produces agitation of the irrigants through acoustic streaming and cavitation [33]. This hydrodynamic activation improves penetration of irrigant into the root canal system [34]. This is in accordance with studies that have shown that activation of the irrigants using endoactivator (sonic activation) has resulted in enhanced sealer penetration [35]. It has shown to remove smear layer and reduce bacterial load. When smear layer is removed efficiently, sealer will penetrate deeper into dentinal tubules and attack the residual microbes thereby providing a fluid tight seal [36].
In this study, it was seen that there was an increase in the sealer penetration after activation of sodium hypochlorite which can be attributed to the opening of the tubules due to the activation process.
Limitation(s)
The ratio between ZnO nanoparticles and AH plus sealer cannot be maintained appropriately in each sample since human error cannot be avoided in such small quantity measurements and mixing.
Conclusion(s)
The addition of nanoparticles increase the tubular penetration of sealers owing to its small particle size and good flow. Also, the use of endoactivator improves the tubular sealer penetration. Modified AH plus showed deeper penetration after activation of the irrigating solutions, followed by that without activation. Thus, sealers modified using nanoparticles can be used in routine practice to improve the results owing to its promising properties but further studies need to be conducted to view the overall scenario.
Author Declaration:
Financial or Other Competing Interests: None
Was Ethics Committee Approval obtained for this study? Yes
Was informed consent obtained from the subjects involved in the study? NA
For any images presented appropriate consent has been obtained from the subjects. NA
Plagiarism Checking Methods: [Jain H et al.]
Plagiarism X-checker: Feb 13, 2020
Manual Googling: May 18, 2020
iThenticate Software: Aug 29, 2020 (19%)
[1]. Haapasalo M, Udnaes T, Endal U, Persistent, recurrent, and acquired infection of the root canal system post-treatmentEndodontic Topics 2003 6(1):29-56.10.1111/j.1601-1546.2003.00041.x [Google Scholar] [CrossRef]
[2]. Chen H, Zhao X, Qiu Y, Xu D, Cui L, Wu B, The tubular penetration depth and adaption of four sealers: A scanning electron microscopic studyBiomed Res. Int 2017 2017:294652410.1155/2017/294652429479539 [Google Scholar] [CrossRef] [PubMed]
[3]. Shenoy VU, Sumanthini MV, Resilon-Epiphany obturating systemJ Contemp Dent 2011 1:30-32.10.5005/jcd-1-1-30 [Google Scholar] [CrossRef]
[4]. AH plus root canal sealer- dentsply maillefer product brochure. http://www.maillefer.com/product/310/ [Google Scholar]
[5]. Wang ZL, Zinc oxide nanostructures: Growth, properties and applicationsJournal of Physics: Condensed Matter 2004 16(25):R829-58.10.1088/0953-8984/16/25/R01 [Google Scholar] [CrossRef]
[6]. Wang J, Mei Q, Lin L, Sun F, Li J, Zou Q, A comparison of the characteristics of polyurethane-based sealers including various antimicrobial agentsRSC Advances 2019 9(13):7043-56.10.1039/C8RA09374A [Google Scholar] [CrossRef]
[7]. Teixeira AB, Vidal CL, de Castro DT, da Costa Valente ML, Oliveira-Santos C, Alves OL, Effect of incorporation of a new antimicrobial nanomaterial on the physical-chemical properties of endodontic sealersJ Conserv Dent: JCD 2017 20(6):392-97.10.4103/JCD.JCD_266_1729430089 [Google Scholar] [CrossRef] [PubMed]
[8]. Gu Y, Perinpanayagam H, Kum DJ, Yoo YJ, Jeong JS, Lim SM, Effect of different agitation techniques on the penetration of irrigant and sealer into dentinal tubulesPhotomed Laser Surg 2017 35(2):71-77.10.1089/pho.2016.412527929924 [Google Scholar] [CrossRef] [PubMed]
[9]. White RR, Goldman M, Lin Ps, The influence of smear layer upon DT penetration by plastic filling materialsJ Endod 1985 10(12):558-62.10.1016/S0099-2399(84)80100-4 [Google Scholar] [CrossRef]
[10]. Helung I, Chandler NP, The antimicrobial effect within DT of four root canal sealersJ Endod 1996 22(5):257-59.10.1016/S0099-2399(06)80144-5 [Google Scholar] [CrossRef]
[11]. Saraf-Dadpe A, Kamra AI, A scanning electron microscopic evaluation of the penetration of root canal dentinal tubules by four different endodontic sealers: A zinc oxide eugenol-based sealer, two resin-based sealers and a Polydimethylsiloxane-based sealer: An invitro studyENDODONTOLOGY 2012 1(20):73-3. [Google Scholar]
[12]. Balguerie E, van der Sluis L, Vallaeys K, Gurgel-Georgelin M, Diemer F, Sealer penetration and adaptation in the dentinal tubules: A scanning electron microscopic studyJ Endod 2011 37(11):1576-79.10.1016/j.joen.2011.07.00522000467 [Google Scholar] [CrossRef] [PubMed]
[13]. De Deus GA, Gurgel-Filho ED, Maniglia-Ferreira C, Coutinho-Filho T, Intratubular penetration of root canal sealersPesq Odonto Bras 2002 16(4):332-36.10.1590/S1517-7491200200040000912612772 [Google Scholar] [CrossRef] [PubMed]
[14]. De Deus GA, Gurgel-Filho ED, Maniglia-Ferreira C, Coutinho-Filho T, The influence of filling technique on depth of tubule penetration by root canal sealer: A study using light microscopy and digital image processingAust Endod J 2004 30(1):23-28.10.1111/j.1747-4477.2004.tb00164.x15116906 [Google Scholar] [CrossRef] [PubMed]
[15]. Ørstavik D, Materials used for root canal obturation: Technical, biological and clinical testingEndod Topics 2005 12(1):25-38.10.1111/j.1601-1546.2005.00197.x [Google Scholar] [CrossRef]
[16]. Bernardes RA, deAmorinCampelo A, Silva S, Pereira LO, Duarte MAH, Moraes IG, Bramante CM, Evaluation of the flow rate of 3endodontic sealers: Sealer 26, AH Plus and MTA obturaJ Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2010 109(1):47-49.10.1016/j.tripleo.2009.08.03820123369 [Google Scholar] [CrossRef] [PubMed]
[17]. Kishen A, Shi Z, Shrestha A, Neoh KG, An investigation on the antibacterial and antibiofilm efficacy of cationic nanoparticulates for root canal disinfectionJ Endod 2008 34(12):1515-20.10.1016/j.joen.2008.08.03519026885 [Google Scholar] [CrossRef] [PubMed]
[18]. Desouky AA, Negm MM, Ali MM, Sealability of different root canal nanosealers: Nano calcium hydroxide and nano bioactive glassOpen Dent J 2019 13(1):308-15.10.2174/1874210601913010308 [Google Scholar] [CrossRef]
[19]. Hanley C, Layne J, Punnoose A, Reddy KM, Coombs I, Coombs A, Preferential killing of cancer cells and activated human T cells using ZnO nanoparticlesNanotechnology 2008 19(29):29510310.1088/0957-4484/19/29/29510318836572 [Google Scholar] [CrossRef] [PubMed]
[20]. Nair S, Sasidharan A, Divya Rani VV, Menon D, Nair S, Manzoor K, Role of size scale of ZnO nanoparticles and microparticles on toxicity toward bacteria and osteoblast cancer cellsJ Mater Sci Mater Med 2009 20(Suppl 1):S235-41.10.1007/s10856-008-3548-518716714 [Google Scholar] [CrossRef] [PubMed]
[21]. Kumar N, Dorfman A, Hahm J, Ultrasensitive DNA sequence detection using nanoscale ZnO sensor arraysNanotechnology 2006 17(12):287510.1088/0957-4484/17/12/009 [Google Scholar] [CrossRef]
[22]. Applerot G, Lipovsky A, Dror R, Perkas N, Nitzan Y, Lubart R, Enhanced antibacterial activity of nanocrystalline ZnO due to increased ROS-Mediated cell injuryAdv Funct Mater 2009 19(6):842-52.10.1002/adfm.200801081 [Google Scholar] [CrossRef]
[23]. Li Q, Chen SL, Jiang WC, Durability of nanoZnO antibacterial cotton fabric to sweatJ Appl Polym Sci 2007 103(1):412-16.10.1002/app.24866 [Google Scholar] [CrossRef]
[24]. Zhang L, Ding Y, Povey M, York D, ZnO nano fluids-A potential antibacterial agentProg Nat Sci 2008 18:939-44.10.1016/j.pnsc.2008.01.026 [Google Scholar] [CrossRef]
[25]. Camps J, Pommel L, Bukiet F, About I, Influence of the powder/liquid ratio on the properties of zinc oxide-eugenol-based root canal sealersDent Mater 2004 20(10):915-23.10.1016/j.dental.2004.02.00215501319 [Google Scholar] [CrossRef] [PubMed]
[26]. Takatsuka T, Tanaka K, Iijima Y, Inhibition of dentine demineralization by zinc oxide: Invitro and in situ studiesDent Mater 2005 21(12):1170-77.10.1016/j.dental.2005.02.00616046230 [Google Scholar] [CrossRef] [PubMed]
[27]. Wong RH, Palamara JE, Wilson PR, Reynolds EC, Burrow MF, Effect of CPP-ACP addition on physical properties of zinc oxide non-eugenol temporary cementsDent Mater 2011 27(4):329-38.10.1016/j.dental.2010.11.01121167585 [Google Scholar] [CrossRef] [PubMed]
[28]. Javidi M, Zarei M, Naghavi N, Mortazavi M, Nejat AH, Zinc oxide nano-particles as sealer in endodontics and its sealing abilityContemp Clin Dent 2014 5(1):2010.4103/0976-237X.12865624808690 [Google Scholar] [CrossRef] [PubMed]
[29]. ElKateb WM, Massoud AG, Mokhless NA, Shalaby TI, Measurement of tubular penetration depth of three types of nanopartcles mixed with endodontic sealer using scanning electron microscope (an invitro study)J Am Sci 2015 11(11):111-22. [Google Scholar]
[30]. Alamoudi RA, The smear layer in endodontic: To keep or remove-an updated overviewSaudi Endod J 2019 9(2):71-81. [Google Scholar]
[31]. Mohammadi Z, Sodium hypochlorite in endodontics: An update reviewInt Dent J 2008 58(6):329-41.10.1111/j.1875-595X.2008.tb00354.x19145794 [Google Scholar] [CrossRef] [PubMed]
[32]. Ruddle CJ, Endodontic disinfection: The sonic advantageDentistry today 2017 36(6):84-86. [Google Scholar]
[33]. Townsend C, Maki J, An invitro comparison of new irrigation and agitation techniques to ultrasonic agitation in removing bacteria from a simulated root canalJ Endod 2009 35(7):1040-43.10.1016/j.joen.2009.04.00719567330 [Google Scholar] [CrossRef] [PubMed]
[34]. Guerisoli DM, Marchesan MA, Walmsley AD, Lumley PJ, Pecora JD, Evaluation of smear layer removal by EDTAC and sodium hypochlorite with ultrasonic agitationInt Endod J 2002 35(5):418-21.10.1046/j.1365-2591.2002.00488.x12059911 [Google Scholar] [CrossRef] [PubMed]
[35]. Bharti R, Tikku AP, Chandra A, Shakya VK, Yadav S, Depth and percentage of resin-based sealer penetration inside the dentinal tubules using EndoVac, EndoActivator, Navi tip FX irrigation system: A confocal laser scanning microscope studyJ Conserv Dent 2018 21(2):216-20.10.4103/JCD.JCD_222_1729674828 [Google Scholar] [CrossRef] [PubMed]
[36]. Kokkas AB, Boutsioukis ACh, Vassiliadis LP, Stavrianos CK, The influence of the smear layer on dentinal tubule penetration depth by three different root canal sealers: An invitro studyJ Endod 2004 30(2):100-02.0.1097/00004770-200402000-0000914977306 [Google Scholar] [CrossRef] [PubMed]