Introduction and Current Status of SARS-CoV-2
Chinese authorities on 29th December, 2019 identified a cluster of ‘pneumonia cases of unknown aetiology’ in Wuhan, Hubei province, China [1] and alerted the World Health Organisation (WHO) on 31st December, 2019. By 7th January, 2020 deep sequencing to detect genetic material had been employed to identify the novel Coronavirus-2019 (nCoV-2019) [2]. However, the definitive source of the virus is still a mystery as the earliest case traced back to 1st December 2019 had no reported link to the seafood market. To be precise, 13 (32%) of the 41 early cases had no link to the seafood marketplace [3]. On 21st January 2020, the WHO also suggested that there was possible sustained human-to-human transmission [4]. On 30th January, 2020 the WHO Director-General declared the outbreak a ‘Public Health Emergency of International Concern’- PHEIC [5,6]. On 11th February, 2020 the virus, was named SARS-CoV-2 and the disease it causes as COVID-19 [7]. On 11th of March, 2020 the WHO Director-General declared the COVID-19 outbreak a ‘Pandemic’ [8]. The median incubation period has been estimated to be ranging from 4.2 days (95% CI 3.5-5.1 days) [9] to 5.1 days (95% CI, 4.5-5.8 days) [10].
Since then, 15,117,078 confirmed cases and 620,033 deaths have been reported due to SARS-CoV-2 infection across the globe till 22nd July 2020 [11]. On 13th January, 2020 Thailand reported the first confirmed case of the novel coronavirus outside China. This was confirmed as an imported case from Wuhan, China [12]. On 23rd January, 2020, USA reported its first case of SARS-CoV-2 and the count rapidly increased to 1,678 on 16th March, 2020. Since then, the number of cases has increased more than 2000 times to 4,028,733 on 22nd July, 2020 [11]. The USA, Brazil, India, Russia, South Africa, Peru, Mexico, Chile, Spain and the United- kingdom are among the ten most affected countries of SARS-CoV-2 infection as on 22nd July, 2020 [11]. In the initial stages of the infection, the intensity of spread seemed low which may be attributed to limited testing for the virus but later it increased exponentially. In this context, it is important to understand the basics of transmissibility and severity of SARS-CoV-2 infection as transmissibility and severity are the two vital factors that determine the scourge caused by an epidemic.
High Transmissibility of SARS-CoV-2
Coronavirus are the large family of viruses first identified in mid-1960s in humans. Four human coronaviruses-OC43, HKU1, NL63, and 229E have been irking mankind for decades, causing mild disease such as common colds while MERS and SARS cause more severe afflictions. The seventh coronavirus to affect humans being-SARS-CoV-2 causes the disease COVID-19. The closest wild comparative of SARS-CoV-2 is found in bats, which suggests its origin in a bat, from where it jumped to humans either directly or through another species [13].
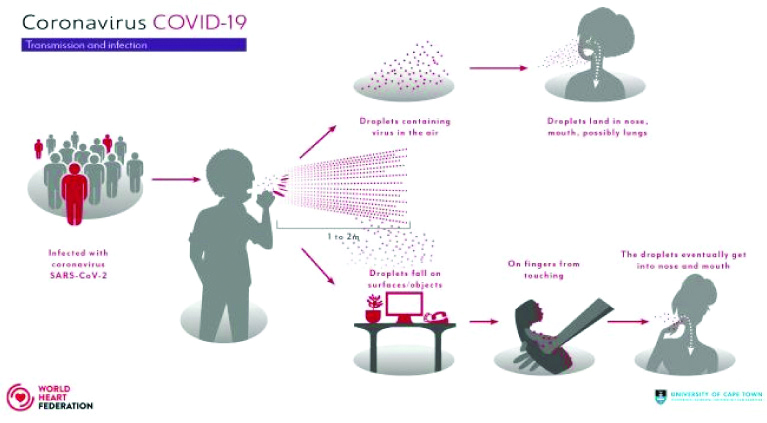
Like SARS-CoV (2003) and MERS-CoV (2012), the SARS-CoV-2 can be transmitted mainly from human to human by respiratory droplets (larger particles >5 μm which travel under 1 m) and contact (e.g., with objects that have been infected with droplets and then touching eyes, nose or mouth). However, researchers at Massachusetts Institute of Technology, Cambridge have suggested possible spread by aerosols (smaller particles <5 μm that travel over 1 m, up to 8 m) [14,15] [Table/Fig-1].
COVID-19 transmission and infection.
Image Source: World Heart Federation [15]
Peculiar Structural Characteristics Facilitating High Transmission of SARS-CoV-2
The SARS-CoV-2 has a crown-like appearance due to the presence of mushroom-shaped proteins (spikes) that protrude from their surface. The spikes bind and fuse to a protein called Angiotensin-converting Enzyme 2 (ACE-2) on human cells, allowing the virus to gain entry. The shape of SARS-CoV-2 spikes facilitates its firm binding to ACE-2 with much higher affinity (10-20 times) than SARS which in turn reduces the amount of virus required to start an infection. Also, the spikes of SARS-CoV-2 show 98% resemblance in sequence with the spike protein of SARS apart from the peculiar furin-like cleavage site in the spikes of the SARS-CoV-2 which are absent in the other SARS-like CoVs [16]. The spike activates when it’s two otherwise connected halves are detached; which is a prerequisite for the virus to enter a host cell. In SARS, this detachment happens with some difficulty but in SARS-CoV-2, the two halves can be easily separated by an enzyme called furin, which is ubiquitously found across many human tissues. These factors might to some extent explain the increased transmissibility of this virus and the evaluation of furin inhibitors as potential anti-viral drugs in the efforts to develop therapeutics for SARS-CoV-2 [13].
Basic Reproduction Number/R naught (R0)
A critical factor that determines the transmissibility is the basic reproduction number R0. Dietz K states that R0 is a measure of average number of secondary cases per case (number of successful transmissions per case) in a totally susceptible population [17]. If R0 is less than one, the number of cases decreases and the disease dies out ‘eventually’. If R0 is equal to one, virus will stay alive and the number of cases will be stable but if R0 is greater than one, the number of cases increase, infections could grow exponentially and could convert into a pandemic. R0 is driven by the duration of contagiousness (host factor), the contact rate between the infected and the susceptible (environmental factor), and the likelihood of infection when contact is made i.e., infectiousness of a pathogen (agent factor). Any factor which influences the contact rate e.g., population density, human social behaviour, biological characteristics of particular pathogens, temperature etc., will ultimately affect R0 [18]. R0 does not consider the transmission heterogeneity among the infected population. Even though two pathogens have the same R0, the transmission patterns may still be different [19]. To bring the R0 down to less is the primary disease control measure. Vaccination eliminates the contact transmission between effected and susceptible population. However, this will not reduce the R0, because the definition of R0 includes the assumption of a completely susceptible population. The effective reproductive number (Rt) for a specific period gives the actual effect of vaccination, as it does not assume complete susceptibility of the population. Thus, it can be estimated in a population having some herd immunity [20]. It can change and be monitored as the number of susceptible members in a population change due to changing behaviours (e.g., physical distancing), treatment and vaccination. However, R0 values are nearly always estimated from mathematical models and the estimated values are dependent on numerous assumptions made in the modeling process.
Severity of SARS-CoV-2
The onslaught of an epidemic depends not only on its basic reproduction number but also its severity which is measured by the CFR.
Case Fatality Rate (CFR) in Recent Epidemics Since the New Millennium
Influenza virus (H1N1) originated in Southern California on 15th April, 2009, was transmitted from human to human and spread to 41 US states and 21 countries [21]. Although, H1N1-2009 was high transmissible, it was less severe with a CFR of 0.03% [Table/Fig-2] [22-25]. Balcan D et al., estimated initial value of R0 as 1.7 for H1N1-2009 which caused 201,200 deaths during the first year [26]. The initial value of R0 in case of SARS-CoV (2003) was greater than 2, indicating a self-sustaining person to person transmission but only 8,098 SARS-CoV cases were reported, and 774 deaths occurred (9.6% CFR) in 37 countries [26]. Cases were mostly detected due to high severity, making quick identification and isolation of individuals possible. However, SARS coronavirus was present in lower concentrations in upper respiratory secretions thereby, explaining its low transmission. Later estimates of R0 were less than 1, showing that self-sustained human to human transmission declined and eventually disappeared [27]. Similarly, MERS-CoV (2012) showed low transmissibility but high severity. MERS-CoV caused 2,494 cases and 858 deaths (34% CFR) in 27 countries. The virus caused rapid outbreaks, mostly in South Korea, Jordan and Saudi Arabia, but the estimated reproduction rate was less than one (R0<1) [28].
Case Fatality Rate (CFR) and R0 value of the commonly known emerging virus infections [22-25].
| Virus | CFR (%) | R0 |
|---|
| SARS-CoV-2 | 0.1-14.6 | 1.4-5.5 [23-25] |
| SARS-CoV (2003) | 10 | 2.5 |
| MERS-CoV (2012) | 40 | <1 |
| Avian H7N9 (2013) | 40 | <1 |
| A H1N1 (2009) | 0.03 | 1.64-1.88 |
| H1N1 (1918) | 3 | 1.4-1.6 |
| Measles virus | 0.3 | 12-18 |
| Rhinovirus | <0.01 | 6 |
| Ebola virus | 70 | 1.5-2.5 |
| HIVb | 80 | 2-4 |
| Small pox virus | 17 | 5-7 |
Sources: This table has been taken from Chen (2020) [22];
b: Without therapy
Case Fatality Rate (CFR) of SARS-CoV-2
A vital concern and challenge in case of SARS-CoV-2 is its R0 which is between 1.4-5.5 [Table/Fig-2] and the estimates of its CFRs vary in different regions of the world [29]. Dictionary of Epidemiology defines CFR as ‘the proportion of cases of a specified condition that are fatal within a specified time [30].
However, CFR in case of SARS-CoV-2 might be wrongly estimated due to a myriad of reasons. It may be underestimated because of a time-lag bias as patients who die on any given day were infected much earlier. Furthermore, calculations are based on false supposition that all cases are being tested and all SARS-CoV-2 deaths are being reported so the full denominator remains largely unknown because asymptomatic cases or patients with very mild symptoms might not be tested at all and the numerator may be deflated due to a large number of undiagnosed or misdiagnosed deaths taking place at home as the public health surveillance system in many countries such as India is not robust enough to count and report mortality from all causes. CFR might also be overestimated due to selection bias as those with severe disease may be preferentially tested and due to the fact that cases might be defined either as total cases (i.e., every confirmed case) or as closed cases (i.e., those with an outcome in the form of recovery or death) [31].
Moreover, there may be other factors that account for mutable mortality rates such as coinfection, comorbidities, disparities in healthcare facilities across continents, age pyramids of different countries e.g., India, being the world’s youngest country might buck the trend vis-a-vis older patients contributing more to overall mortality rates in Italy. Differences may also be attributed to how deaths are ascribed to Coronavirus: dying with the disease (association) or dying from the disease (causation). A review of the available literature leads us to a global CFR of 4.1%, ranging from lowest of 0.06% in Singapore to 2.5% in India, 3.6% in the US, 15.3% in Belgium (their estimated case fatality includes not only confirmed deaths due to SARS-COV-2 but even those suspected of being linked irrespective of whether the victim was tested or not), 17% in France to a maximum of 28% in Yemen as on 22nd July 2020 [32]. CFR is being calculated using different formulas in different settings, such as total deaths divided by total confirmed cases; dividing the total number of deaths by the total number of confirmed cases 14 days previously etc., [33]. Estimating CFR in the early stage of an outbreak is riddled with uncertainties and the estimates are likely to change with time as in case of SARS 2003 epidemic where while the epidemic was still ongoing, the WHO reported a CFR of 4% whereas the final CFR was estimated to be 9.6% [11].
Final Viewpoint
At this point when the pandemic is nowhere near its tail end, it would not be wrong to state that given the fact that most of the cases are asymptomatic or mildly symptomatic, the denominator in CFR estimations is missing millions of untested cases which when added would reduce the CFR to fractions even after accounting for a large number of unreported deaths due to SARS-CoV-2. Therefore, the authors of this paper reiterate that as SARS-CoV-2 spread continues unabated globally, paralleled with synchronised efforts by all governments to ramp up their testing capacities, as more and more data becomes available from country-specific cohorts, it should certainly mitigate the case fatality risk of this new disease to fractions, i.e., eventually, the CFR of COVID-19 even in India may not be 2.5/100 but more like 2.5/1,000 or even 2.5/10,000.
Possible Scenarios of the SARS-CoV-2 Pandemic
In midst of the incertitude vexing epidemiologists, immunologists, clinicians, disease modelers and politicians these possible scenarios in the timeline of events are thinkable: 1) It might never end in the sense that the virus might become endemic, there may be secondary peaks when lockdowns end or there may be sporadic outbreaks or it may keep reappearing seasonally; 2) Aggressive advocacies of public health and hygiene measures, continued social distancing etc., (till we have an effective vaccine or therapeutic agent), succeeds in lowering the contact rate and keeping R0 below one which shrinks the epidemic; 3) As it silently infects more and more people, herd immunity develops and it ends like a forest fire with no more susceptibles left; 4) An effective vaccine makes the population immune and the virus amenable to eradication.
Conclusion(s)
Gaping holes in our information regarding the natural history, agent, host and environmental factors need to be bridged by future studies as they affect both transmission and severity of SARS-CoV-2. There are a host of factors to study, age and gender being the most significant of all. The geriatric population is consistently showing high severity possibly because of associated comorbities and their debilitated immune systems which fail to mount an effective response to a novel infection, while children and females are less affected. Other factors like vaccination for diseases like tuberculosis, presence of other autoimmune disorders, duration of infectiousness of cases (average time for which a patient keeps shedding the virus), probability of infection being transmitted during contact between a susceptible and infected individual, the amount of virus they’re exposed to along with effect of temperature and humidity might also play a role in transmission and are under investigation.
In the absence of a clear road map, the answers to these queries are eagerly awaited in case of the COVID-19 pandemic and every single day counts. Presently, the virus in its naivety, has waged a war on this planet of immunologically susceptible people and unless people can slow the spread of the virus by strictly sticking to age old recommendations of hand hygiene, physical-distancing and universal masking of faces, the wait for a medical breakthrough in the form of an effective vaccine or a therapeutic agent would inevitably be fraught with pain and distress for every single life lost in desolation counts.
[1]. Tan W, Zhao X, Ma X, Wang W, Niu P, Xu W, A novel coronavirus genome identified in a cluster of pneumonia cases- Wuhan, China 2019-2020China CDC Weekly 2020 2(4):61-62.10.46234/ccdcw2020.017 [Google Scholar] [CrossRef]
[2]. WHO Coronavirus disease (COVID-2019) situation reports https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situationreport [Google Scholar]
[3]. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Clinical features of patients infected with 2019 novel coronavirus in Wuhan, ChinaThe Lancet 2020 395(10223):497-506.10.1016/S0140-6736(20)30183-5 [Google Scholar] [CrossRef]
[4]. World Health Organisation Western Pacific, on Twitter, 2020 Assessed on 10th April, 2020 [Google Scholar]
[5]. World Health Organisation. 2019-nCoV outbreak is an emergency of international concern. http://www.euro.who.int/en/health-topics/health-emergencies/international-health-regulations/news/news/2020/2/2019-ncov-outbreak-is-an-emergency-of-international-concern [Google Scholar]
[6]. Statement on the second meeting of the International Health Regulations (2005) Emergency Committee regarding the outbreak of novel coronavirus (2019-nCoV) https://www.who.int/news-room/detail/30-01-2020- statement-on-the-second-meeting-of-the-international-health-regulations-(2005)- emergency-committee-regarding-the-outbreak-of-novel-coronavirus-(2019-ncov) [Google Scholar]
[7]. WHO. Naming the coronavirus disease (COVID-19) and the virus that causes it. https://www.who.int/emergencies/diseases/novel-coronavirus2019/technical-guidance/naming-the-coronavirus-disease-(covid-2019)-and-thevirus-that-causes-it [Google Scholar]
[8]. WHO Director-General’s opening remarks at the media briefing on COVID-19-11 March 2020 https://www.who.int/dg/speeches/detail/who-directorgeneral-s-opening-remarks-at-the-media-briefing-on-covid-19-2020 [Google Scholar]
[9]. Sanche S, Lin YT, Xu C, Romero-Severson E, Hengartner N, Ke R, High contagiousness and rapid spread of severe acute respiratory syndrome coronavirus 2Emerg Infect Dis 2020 26(7)10.3201/eid2607.20028232255761 [Google Scholar] [CrossRef] [PubMed]
[10]. Lauer SA, Grantz KH, Bi Q, Jones FK, Zheng Q, Meredith HR, The incubation period of coronavirus disease 2019 (covid-19) from publicly reported confirmed cases: Estimation and applicationAnnals of Internal Medicine 2020 172(9):577-82.10.7326/M20-050432150748 [Google Scholar] [CrossRef] [PubMed]
[11]. https://www.worldometers.info/coronavirus/coronavirus-death-rate/Accessed on on 22 July, 2020 [Google Scholar]
[12]. WHO Coronavirus disease (COVID-2019) situation reports https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situationreports [Google Scholar]
[13]. https://www.theatlantic.com/science/archive/2020/03/biography-new-oronavirus/608338/. Assessed on 2 April, 2020 [Google Scholar]
[14]. Bourouiba L, Turbulent gas clouds and respiratory pathogen emissions: Potential implications for reducing transmission of COVID-19JAMA 2020 Mar 26 10.1001/jama.2020.475632215590 [Google Scholar] [CrossRef] [PubMed]
[15]. https://www.world-heart-federation.org/resources/covid-19-transmission/Accessed 16 July, 2020 [Google Scholar]
[16]. Coutard B, Valle C, de Lamballerie X, Canard B, Seidah NG, Decroly E, The spike glycoprotein of the new coronavirus 2019-nCoV contains a furin-like cleavage site absent in CoV of the same cladeAntiviral Research 2020 176:10474210.1016/j.antiviral.2020.10474232057769 [Google Scholar] [CrossRef] [PubMed]
[17]. Dietz K, The estimation of the basic reproduction number for infectious diseasesStatistical Methods in Medical Research 1993 2(1):23-41.10.1177/0962280293002001038261248 [Google Scholar] [CrossRef] [PubMed]
[18]. Delamater PL, Street EJ, Leslie TF, Yang YT, Jacobsen KH, Complexity of the basic reproduction number (R0)Emerg Infect Dis 2019 25(1):110.3201/eid2501.17190130560777 [Google Scholar] [CrossRef] [PubMed]
[19]. Prevent Epidemics. COVID-19 Weekly Science Review. https://preventepidemics.org /coronavirus/science-review/april-4-10-2020/ [Google Scholar]
[20]. Anderson RM, The concept of herd immunity and the design of community-based immunization programmesVaccine 1992 10(13):928-35.10.1016/0264-410X(92)90327-G [Google Scholar] [CrossRef]
[21]. https://www.cdc.gov/h1n1flu/cdcresponse.htm. Assessed on 22, July 2020 [Google Scholar]
[22]. Chen J, Pathogenicity and transmissibility of 2019-nCoV- A quick overview and comparison with other emerging virusesMicrobes and Infection 2020 22(2):69-71.10.1016/j.micinf.2020.01.00432032682 [Google Scholar] [CrossRef] [PubMed]
[23]. WHO, Statement on the meeting of the International Health regulations (2005) Emergency Committee regarding the outbreak of novel coronavirus (2019-nCoV). Assessed on July 22, 2020 [Google Scholar]
[24]. Zhao S, Lin Q, Ran J, Musa SS, Yang G, Wang W, Preliminary estimation of the basic reproduction number of novel coronavirus (2019-nCoV) in China, from 2019 to 2020: A data-driven analysis in the early phase of the outbreakInt J Infect Dis 2020 92:214-17.10.1016/j.ijid.2020.01.05032007643 [Google Scholar] [CrossRef] [PubMed]
[25]. Read JM, Bridgen JR, Cummings DA, Ho A, Jewell CP, Novel coronavirus 2019-nCoV: early estimation of epidemiological parameters and epidemic predictionsMed Rxiv 2020 https://doi.org/10.1101/2020.01.23.2001854910.1101/2020.01.23.20018549 [Google Scholar] [CrossRef]
[26]. Balcan D, Hu H, Goncalves B, Bajardi P, Poletto C, Ramasco JJ, Seasonal transmission potential and activity peaks of the new influenza A (H1N1): A Monte Carlo likelihood analysis based on human mobilityBMC Medicine 2009 7(1):4510.1186/1741-7015-7-4519744314 [Google Scholar] [CrossRef] [PubMed]
[27]. Chowell G, Castillo-Chavez C, Fenimore PW, Kribs-Zaleta CM, Arriola L, Hyman JM, Model parameters and outbreak control for SARSEmerg Infect Dis 2004 10(7):125810.3201/eid1007.03064715324546 [Google Scholar] [CrossRef] [PubMed]
[28]. Killerby ME, Biggs HM, Midgley CM, Gerber SI, Watson JT, Middle East respiratory syndrome coronavirus transmissionEmerg Infect Dis 2020 26(2):19110.3201/eid2602.19069731961300 [Google Scholar] [CrossRef] [PubMed]
[29]. Spychalski P, Błażyńska-Spychalska A, Kobiela J, Estimating case fatality rates of COVID-19Lancet Infect Dis 2020 20(7):774-75.10.1016/S1473-3099(20)30246-2 [Google Scholar] [CrossRef]
[30]. Porta M, A dictionary of epidemiology 2008 5th ednOxfordOxford University Press [Google Scholar]
[31]. Pueyo T Coronavirus: why you must act now. Available from:https://medium.com/@tomaspueyo/coronavirus-act-today-or-people-will-die-f4d3d9cd99ca [Google Scholar]
[32]. https://ourworldindata.org/grapher/coronavirus-cfr?tab=table&country= BRA~ DEU~ ISL~ IND~ITA~KOR~ESP~USA~OWID_WRL Accessed 22, July, 2020 [Google Scholar]
[33]. Baud D, Qi X, Nielsen-Saines K, Musso D, Pomar L, Favre G, Real estimates of mortality following COVID-19 infectionLancet Infect Dis 2020 20(7):77310.1016/S1473-3099(20)30195-X [Google Scholar] [CrossRef]