Despite the use of advanced antibiotics and resuscitation therapies, sepsis remains a prime cause of death in critically ill patients [1]. The highly variable and non-specific nature of the signs and symptoms makes the diagnosis and evaluation of severity of sepsis more complicated [2]. One of the important challenges that doctors have to face while treating critically ill patients is sepsis. This is due to delay in diagnosis, which causes late administration of antibiotics resulting in an increase in the mortality in this cohort [3]. Population of India is over 1.3-1.4 billion [4], having more susceptibility towards infectious diseases in the world. Limited availability of diagnostic assays [5], makes the diagnosis more difficult in various infectious diseases. Apart from culture, biomarkers like White Blood Cell (WBC) count, Lactate, C- Reactive Protein (CRP) can also detect sepsis but with less specificity. Biomarkers, primarily from the blood, increase in the early phase of the inflammatory response unlike microbial culture [6]. New biomarkers have been proposed to diagnose sepsis day in and day out, but at present they adequate specificity and sensitivity [7]. In addition to this, antibiotic resistance is rising day by day in India, hence there is a need of specific biomarkers to govern antibiotic dose. There are many studies available on evaluation of PCT in different clinical conditions, which includes even sepsis. PCT is a well proved marker in several studies out of the country but unfortunately, very few studies exist in Indian literature. As the expression of serum PCT is dependent on the genetic constituents of the population, it becomes essential to verify its efficacy as a marker of sepsis in Indian population.
Hence, in India evaluation of proper biomarkers for sepsis along with PCT is needed. So, the quest of finding a good marker which can diagnose sepsis at earliest continues. During sepsis various inflammatory intercessors such as Prostaglandins, Interleukins, Nitric oxide etc., are produced by different activated cells. In the last few years, importance of Arginase as a marker of immunity has increased exceptionally due to the fact that the enzyme is crucially involved in various aspects of inflammation [3,8]. Keeping these facts in mind PCT and Arginase were evaluated as biomarkers for early diagnosis of sepsis.
Materials and Methods
This hospital based, case-control study was conducted from May 2012- July 2015 in the Department of Biochemistry, BJ Medical College is affiliated with Sassoon General Hospital (Pune, Maharashtra, India). The approval from Institutional Ethical Committee was obtained (Ref No: BJMC/IEC/Pharmac/D1210139-41). Hundred adult patients irrespective of sex, admitted in medical and surgical ICU, were screened for signs of SIRS and were incorporated in the study from Sassoon General Hospitals, Pune, Maharashtra, India. Samples were collected after the admission to the ICU. The written consent was taken from the patients before collection of samples. Sample size was calculated based on sensitivity and prevalence:
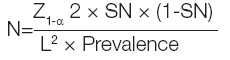
Samples of 100 age and sex matched healthy controls were taken from hospital staff.
Inclusion criteria: Hundred adult patients (age>18 years) that presented more than one of the four clinical signs of SIRS were included in the study. The clinical criteria for SIRS are as follows [10]:
Body temperature greater than 38°C or less than 36°C
Heart rate greater than 90 beats/min
Respiratory rate greater than 20 breaths/min or hyperventilation with a PaCO2 less than 32 mmHg,
WBC count >12000/mm3, <4000/mm3, or with >10% immature neutrophils.
Exclusion criteria: Paediatric patients, patients with pulmonary and liver disorders, chronic diabetic, hypertensive and patients with nephropathy were eliminated.
Sample Collection
A 5 mL of intravenous blood was collected, centrifuged at 3000 rpm for 15 minutes and serum was stored at -80°C till the analysis was done. Activity of arginase was estimated by spectrophotometric method in terms of ornithine formed by Roman and Ray method [11]. Estimation of PCT by ELISA method (SEA689Hu 96 Tests. Enzyme-linked Immunosorbent Assay Kit). This assay has high sensitivity and excellent specificity for detection of PCT.
Statistical Analysis
Data analysis was done using SPSS version 21.0. Results are presented as mean±SD. Unpaired t-test was done to compare the mean biomarker levels between the cases and controls. The AUC was calculated using ROC. The. Specificity and Sensitivity, Positive Predictive Value (PPV), Negative Predictive Value (NPV) and accuracy was calculated for the biomarkers. All the data analysis was set at 95% CI with p<0.05. All values below p<0.05 means the values are statistically significant.
Results
[Table/Fig-1] shows comparison of arginase activity and levels of PCT in sepsis patients with controls (N=100). The study results showed significantly increased levels of both the biomarkers in cases as compared to controls (p<0.01).
Comparison between cases and controls (N=100).
| Biomarker | Group | Mean | SD | p-value |
|---|
| Arginase (IU/L) | Case | 36.8632 | 19.98233 | <0.01** |
| Control | 2.7245 | 1.34726 |
| Procalcitonin (ng/mL) | Case | 2.5410 | 1.54319 | <0.01** |
| Control | 0.0212 | 0.01301 |
**Statistically significant at the 0.01 level (2-tailed)
ROC curves were plotted to find out the AUC and the cut-offs of arginase and PCT for early diagnosis of sepsis. All the values for the ROC were within AUC of 1, and the cut-off point that could be used for early diagnosis of sepsis (SIRS+infection=sepsis) was 0.04 [Table/Fig-2].
ROC for Arginase and Procalcitonin (PCT).
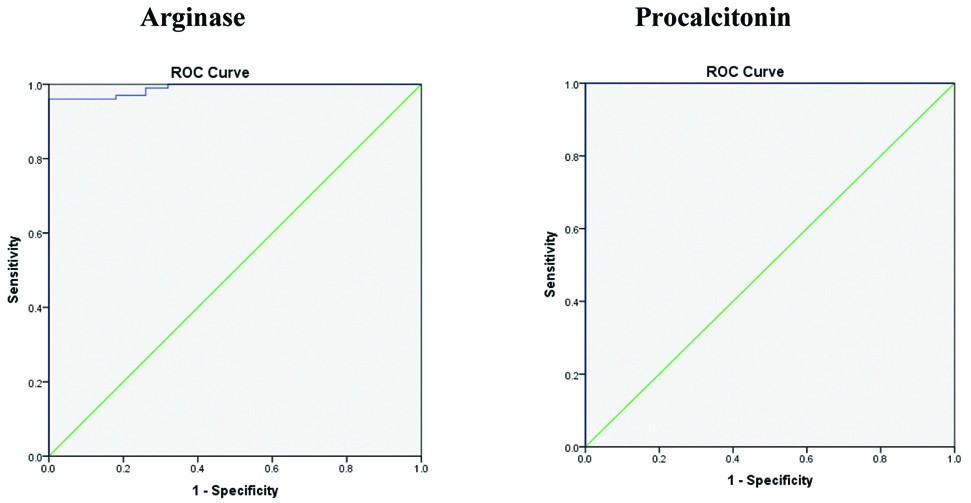
Sensitivity of PCT as a diagnostic test was 100% which means it correctly detects the disease 100 times. Specificity is 82% which means the diagnostic test can correctly detect that there is no disease and those patients were correctly identified [Table/Fig-3].
Table of sensitivity, specificity, PPV, NPV, AUC and Cut-off values for early diagnosis of sepsis.
| Marker | Sensitivity | Specificity | PPV | NPV | Accuracy | AUC | Cut-off |
|---|
| Arginase | 98.95% | 96.12% | 96% | 99% | 97.50% | 0.990 | 4.6 IU/L |
| PCT | 100% | 82% | 84.75% | 100% | 91% | 1.000 | 0.04 ng/mL |
PPV is 84.75%, means PCT has 84.75% probability of showing that the patient has disease when the test is positive. NPV is 100%, means PCT has 100% probability of showing that the patient does not have disease when the test is negative [Table/Fig-3]. Sensitivity of Arginase as a diagnostic test is 98.95% which means it correctly detects the disease 98.95 times. Specificity is 96.12% means the diagnostic test can correctly detect that there is no disease. PPV is 96%, means arginase has 96% probability of showing that the patient has disease when the test is positive. NPV is 99%, means arginase has 99% probability of showing that the patient does not have the disease when the test is negative [Table/Fig-3].
Discussion
Sepsis is defined as the presence of SIRS and a presumed or confirmed infection. There is a great opportunity for well-designed studies which can assess the role of biomarkers for sepsis in India [12]. In the present study, estimation of enzyme Arginase and PCT levels were done to find out whether these markers can be used as diagnostic markers for early detection of sepsis. PCT is the peptide precursor of calcitonin. The para-follicular C cells of the thyroid gland synthesises calcitonin from precursor PCT and regulates calcium homeostasis. Calcitonin is synthesised by the thyroid gland only, in contrast PCT is produced by many cells and organs in a response to different bacterial infections. When the infection sets in PCT level in the serum increases rapidly, usually within 2-6 hours and reaches to the peak in 12 hours. It is not easily degradable as its half-life is 24 hours [13]. As the half-life of PCT is longer than half lives of Tumour Necrosis Factor alpha (TNFα) and Interleukin-6 (IL6) it is suitable as a rapid diagnostic test and for monitoring the progression of disease [14].
In the present study, PCT levels were very high in comparison with controls, (p<0.01) [Table/Fig-1]. With the methodology used in this study, the sensitivity of PCT as a diagnostic test was 100%, and the specificity was 82%. The PPV was 84.75% and NPV 100%, and the accuracy of PCT was 91% [Table/Fig-3]. All these values of PCT indicate that it is a good marker. Normally, serum PCT is cleaved by a specific protease to Katacalcin, N-terminal residue and calcitonin. Usually, all the serum PCT is hydrolysed, and not at all detected in the bloodstream. Once into the circulation, plasma enzymes are unable to breakdown PCT hence, it remains unchanged with a half-life of 25-30 hours which is very long as compared to the half-lives of cytokines like TNF-α and IL-6, which are relatively short. Therefore, in healthy individuals’ serum PCT levels are <0.1 ng/mL. In different types of infections, circulating levels of calcitonin precursors, including PCT and excluding mature Calcitonin (CT), are increased by thousand-fold. This increase and especially the progression of the disease correlates with the severity of the disease hence with mortality [15]. A microbial infection induces the expression of CALC-I gene and also responsible for constitutive release of PCT from all parenchymal tissues and differentiated cell types throughout the body. Hence, under septic conditions, the whole body can be regarded as being an endocrine gland. Tissues like liver, lung, kidney, adipocytes and muscle including parenchymal cells provide the primary source of circulating PCT in sepsis. Greater induction of PCT, mRNA and PCT peptide release occurs from parenchymal cells as compared to circulating cells. This indicates a tissue based and not a leukocyte-based mechanism of host defense. In sepsis, the predominance of PCT in contrast to mature CT is suggestive of a constitutive pathway in cells lacking secretion granules. This means that many enzymatic processing is bypassed [16].
PCT is a biomarker that shows greater specificity than other pro-inflammatory markers in detecting patients with sepsis and can be useful in the diagnosis of bacterial infections. Hence, there is an increased use of PCT measurements for detecting systemic bacterial infections. Patients with bacterial infections showed significant rise in serum PCT, but when patients were infected with a virus or even a serious virus, there was just a slight change in the serum PCT levels [14]. A study found an increase in PCT levels is negligible in viral infections, whereas levels increase subsequently after a single injection of endotoxin [15]. As stated above, a significant rise (p<0.01) observed in PCT levels in the present study is indicative of sepsis. The rise may be due to increased production of PCT by parenchymal cells in response to sepsis. PCT is detectable in blood within 3-4 hours after infection whereas culture tests take about 24-48 hours. It is clear that PCT is a sensitive marker for the early diagnosis of sepsis as compared to culture results. The cut-off obtained from ROC analysis (0.04) can be very useful in early diagnosis of sepsis in the Indian population. In addition, if serial measurements of PCT levels are done then they can be useful in preventing the overuse of antibiotics and avoiding antibiotic resistance. Arginase, the second biomarker of the study, is an enzyme which hydrolyses L-Arginine to the products L-ornithine and urea to protect against toxicity of ammonia. In mammals, arginase exists in 2 isoforms, arginase I (liver) and arginase II (non-hepatic type). They catalyse identical biochemical reactions but vary in cellular expression, regulation, and subcellular localisation [17]. The isoform arginase I (in liver) is one of the enzymes of the urea cycle, which detoxifies ammonia. Arginase II, a mitochondrial protein which is present in various peripheral tissues, such as kidney, prostate, small intestine and the lactating mammary gland. Due to the generation of ornithine, this isoform is involved in many metabolic pathways and has a role in stimulating cell growth and other physiological functions. In recent years arginase is being used as a marker of immunity due to its significant involvement in different aspects of inflammation [3,8].
In the study, arginase values of cases were compared with controls. Arginase levels were very high as compared to controls, (p<0.01) [Table/Fig-1]. Darcya CJ et al., reported in their stable-isotope infusion study that sepsis patients have a higher conversion rate of whole-body L-arginine to urea as compared to controls, resulting in increased total body arginase activity [18]. This finding is in accordance with increased arginase activity of present study. All the values for the diagnosis ROC were within AUC of 0.990, and the cut-off point that could be used for early diagnosis of sepsis was 4.6 IU/L. With the methodology used in this study, the sensitivity of arginase as a diagnostic test was 98.95%, and the specificity was 96.12%. The PPV was 96% and NPV 99%, and the accuracy of arginase was 97.50% [Table/Fig-2]. In the present study, ROC analysis and the cut-off that can be useful for diagnosis of sepsis. As per the knowledge, the authors did not find any such cut-off in the available literature. All these observations can be justified in the following way. Arginine is a semi-essential amino acid in normal health but may become conditionally essential in stressed conditions like sepsis. Sepsis is a systemic response to an infection. Metabolic changes in infection and sepsis could be directly related to changes in the metabolism of L-arginine. In sepsis, arginine catabolism is markedly increased via the arginase and nitric oxide pathways [19]. Protein breakdown is also increased, in order to maintain arginine availability as there is reduction in endogenous arginine production from citrulline and food intake both.
Chang CI et al., postulated that, arginase competes with Nitric Oxide Synthase (NOS) for arginine as it is a common substrate, hence can inhibit Nitric Oxide (NO) production [20]. This controlling mechanism may be important when the supply of extracellular L-arginine is inadequate. Miki K et al., suggested that there is an interplay between arginase and NOS in various cells including immune cells [21]. So, it is the bioavailability of arginine that decides the pathway for the metabolism of arginine and its fate. Das P et al., observed that various pathogens modulate the expression and function of the arginase isoforms, which causes induction of arginase activity [22]. In other words, pathogens evade the immune response. This in turn decreases bactericidal NO and increased polyamine or proline synthesis. Thus, increased arginase activity benefits the host by decreasing the deleterious effects of NO. In addition to this the toxic waste product ammonia is totally removed by urea formation. Arginase being an inflammatory marker the values of arginase increase at an early onset of infection. In the present study increased values of arginase support this statement. Significant increase in levels of arginase (p<0.01) at an early onset of infection indicates that it can be used as a good marker for early diagnosis of sepsis.
Limitation(s)
The present study was a single centre study and so a multicentered study population can commit to the importance of serum PCT and Arginase in critical care. Further studies with a larger sample size can be undertaken to establish the role of arginase and PCT as diagnostic markers and also to establish these markers for early diagnosis of sepsis.
Conclusion(s)
This study has many important applications in clinical practice. Increased values for both PCT and arginase as compared to controls were observed. Sensitivity and specificity of PCT was 100% and 82%, respectively and that of arginase was 98.95% and 96.12%. This means that these markers not only show significant increase in sepsis but they are sensitive and specific also. The cut-offs of PCT and arginase was 0.04 ng/mL and 4.6 IU/L, respectively. PPV and NPV values of both markers also help in proving their efficacy as equally good markers for early diagnosis of sepsis. The study definitely indicates that serum PCT and arginase can be helpful in the management of sepsis in critical care. Serum PCT and arginase offers a high level of accuracy than other currently available tests.
**Statistically significant at the 0.01 level (2-tailed)