Gastrointestinal Stromal Tumours (GISTs) are the most common mesenchymal tumours of the gastrointestinal tract commonly arising from stomach and small intestine. Duodenal GISTs are rare. Though uncommon, GISTs can arise from the omentum, mesentery, gallbladder as well as retroperitoneum and are defined as Extra GISTs (EGIST). Authors have reported a case of large GIST arising from the second part of the duodenum and mimicking the large pancreatic head mass in a 34-year-old female patient presented with abdominal pain and vomitings. The Contrast Enhanced Computed Tomography (CECT) revealed a large enhancing mass in the pancreatico-duodenal groove not separated from pancreatic head. Whipple’s surgery was performed. Histopathology and Immuno-histochemistry report revealed the duodenal GIST with pancreatic infiltration with high risk for metastases according to mitotic index and size. Sometimes large duodenal GISTs can make the diagnosis difficult radiologically due to invasion of pancreatic head and mimic as primary pancreatic head tumour.
Case Report
A 34-year-old female patient was referred to the Department of Radiodiagnosis for CECT abdomen and pelvis from the Liver Transplant and Hepatopancreaticobiliary (HPB) Department. She had presented with abdominal pain, vomitings and heaviness since 3 months. There was no significant medical or surgical history. Ultrasound Sonography (USG) and CT scan performed in other hospital reported an exophytic hepatic mass and hence was referred. Her clinical examination, Liver Function Tests (LFTs) and serum tumour markers were normal.
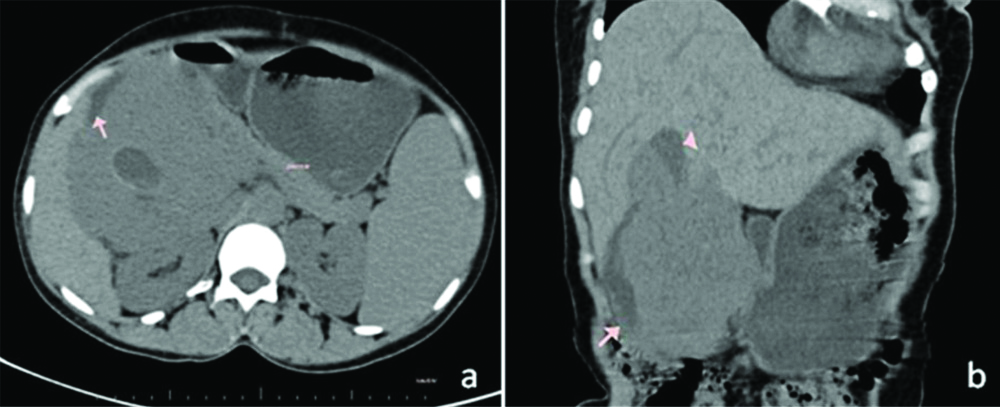
Contrast enhanced Computed Tomography was performed which revealed 11.8×9.9×13.8 cm sized, large heterogeneously hypo to isodense mass lesion in the pancreaticoduodenal groove with few necrotic areas on unenhanced phase. The lesion was not seen separate from the pancreatic head and serosal surface of medial wall of second part of the duodenum [Table/Fig-1]. A small central cystic area with blood-fluid level suggestive of intratumoural haemorrhage was also seen. Superolaterally, the lesion extended upto inferomedial surface of the liver with indistinct fat planes and pressure effect on the gall bladder [Table/Fig-1]. The enhancement was seen predominantly in the arterial phase with dilated gastroduodenal, superior pancreatico-duodenal arteries and few tortuous collateral channels from the right hepatic artery [Table/Fig-2]. The lesion drained through multiple channels into the superior mesenteric vein and main portal vein with their resultant dilatation [Table/Fig-2]. The bulk of the tumour was seen postero-lateral to the Common Bile Duct (CBD) [Table/Fig-3,4] with mild prominence of proximal CBD and pancreatic duct. As a result mild splenomegaly was also noted. No enlarged regional lymph nodes, hepatic metastases or intra-abdominal deposits were identified. Rest of scan was unremarkable. Exact origin of the mass lesion either duodenal or pancreatic head was difficult to ascertain, so imaging differentials of GIST, Non-functional Neuroendocrine Tumour (NET) and Solid Pseudopapillary Epithelial Neoplasm (SPEN) of the pancreas were given. Fine Needle Aspiration (FNA) or biopsy was not performed to avoid the risk of tumour seeding and bleeding.
(a,b): Non contrast images in axial and coronal planes showing the predominantly solid lesion with few cystic areas in the pancreaticoduodenal groove, not seen separate from the D2 of duodenum (arrow) and medially pancreatic head.
GB: Gall bladder
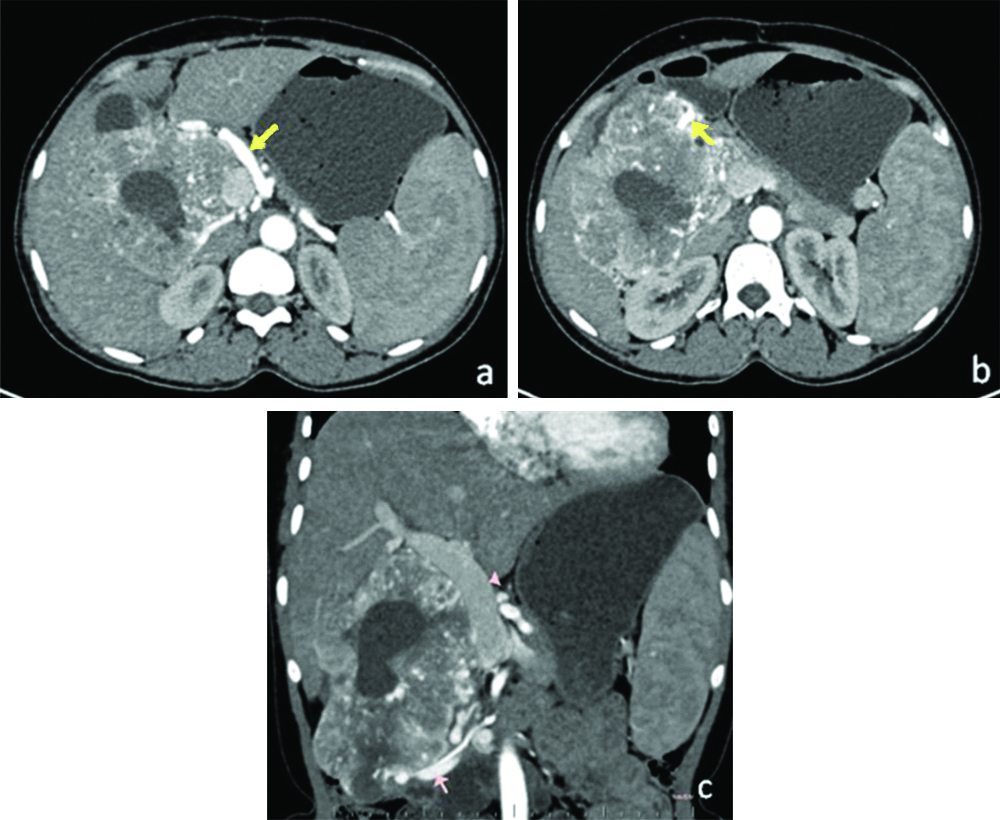
(a, b): Axial images in arterial phase showing enlarged common hepatic (arrow in a), gastroduodenal arteries (arrow in b) with their branches supplying the tumour and early filling of portal venous system suggesting intratumoural shunting. Pancreatic head- arrowhead in b. (c): Coronal image in arterial phase showing dilated and early contrast filled portal vein and SMV. Splenomegaly was also noted.
SMV: Superior mesenteric vein
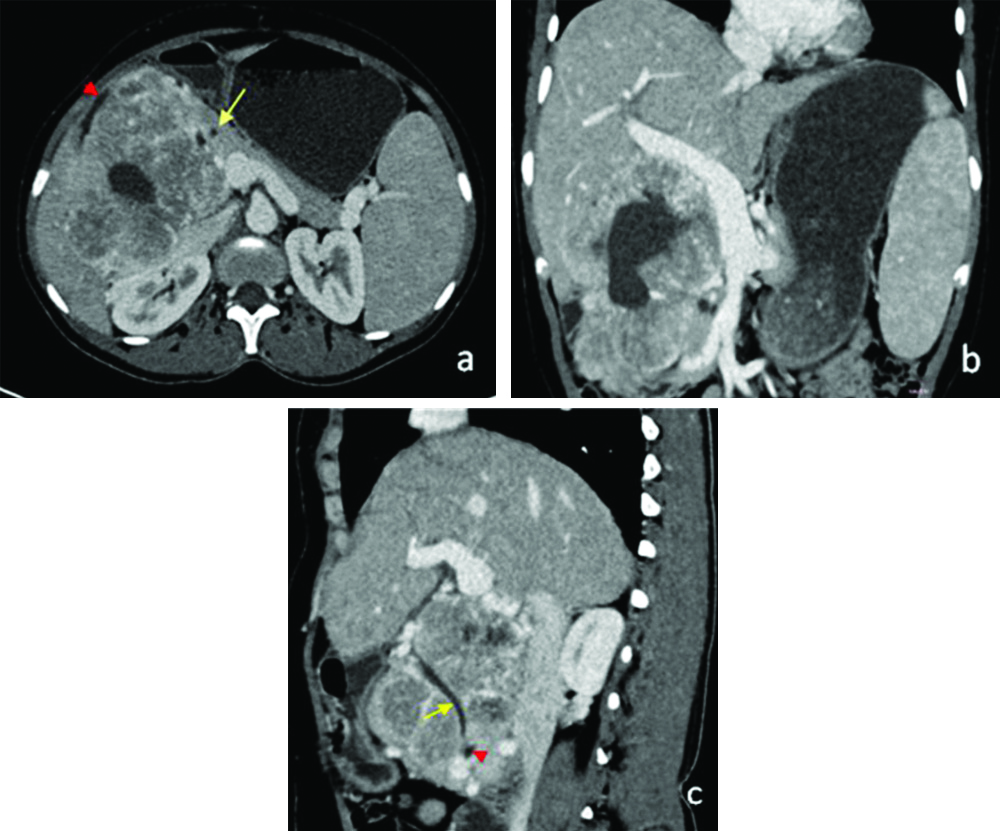
Portal phase axial (a) image revealing tumour inseparable from D2 (arrowhead) and pancreatic head (arrow). Coronal image (b) with dilated portal vein. (c): Sagittal images showing CBD (arrow) traversing through the lesion and pancreatic duct (arrowhead).
CBD: Common bile duct
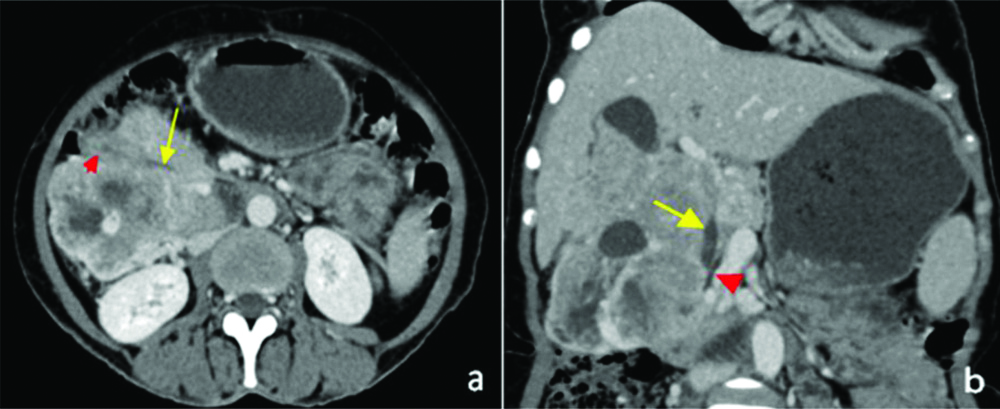
(a,b): Venous phase axial image revealing the opening of CBD (long arrow) in the D2 (arrowhead in a). Pancreatic duct has been shown in image b as an arrowhead.
CBD: Common bile duct
Positron Emission Tomography-Computed Tomography (PET-CT) scan was performed which was negative for metastases. Therefore, after discussion in the multidisciplinary team, the decision of resection of the lesion with Whipple’s surgery was planned. During surgery, 15 x 10×7 cm size mass lesion was identified adjacent to inferior surface of liver, duodenum (D1,D2) and porta superiorly, medially involving head of pancreas and inferiorly extending upto inferior vena cava, right renal hilum. Reconstruction with pancreatico-jejunostomy, hepatico-jejunostomy and gastro-jejunostomy was performed. The specimen was sent to histopathological examination.
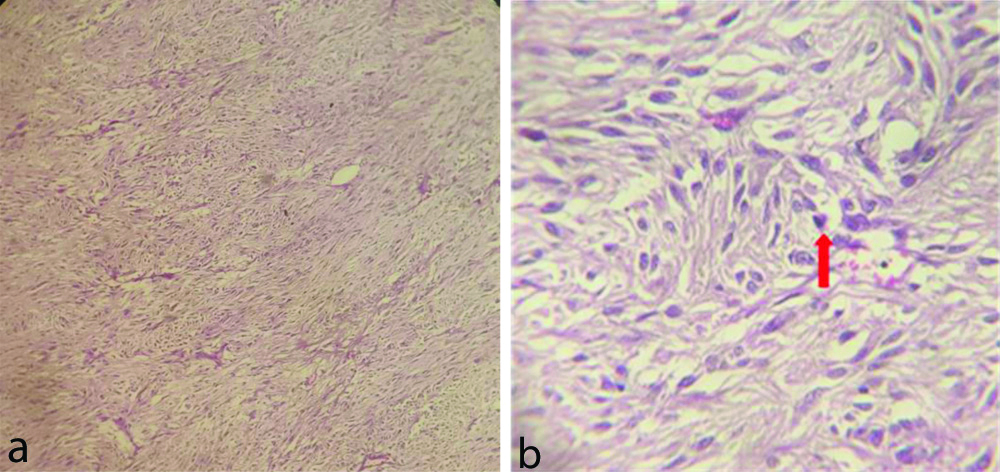
On gross pathology, a tumour was seen predominantly involving head of pancreas and wall of duodenum and cut section revealed solid, grey white appearance. Microscopy revealed tumour arising from the muscular layer of duodenum with infiltration into the pancreatic head. It consisted of ovoid and spindle shaped tumour cells [Table/Fig-5] with hyperchromatic nuclei and moderate pleomorphism [Table/Fig-5,6]. Less than 5/50 High Power Field (HPF) mitosis was observed. Accordingly, the patient was having high metastatic risk as the tumour size >10 cm and mitosis <5/50 HPF. On Immunohistochemistry (IHC), The tumour cells expressed CD34 and DOG1 with weak expression of CD117/ c-KIT and Mib1 labeling index of 15%.
a) Histopathology images with Haematoxylin and Eosin (H&E) showing spindle shaped tumour cells in low power field (40x); b) High Power Field (HPF) (400x magnification) image on the right showing nuclear pleomorphism and a mitotic figure (arrow).
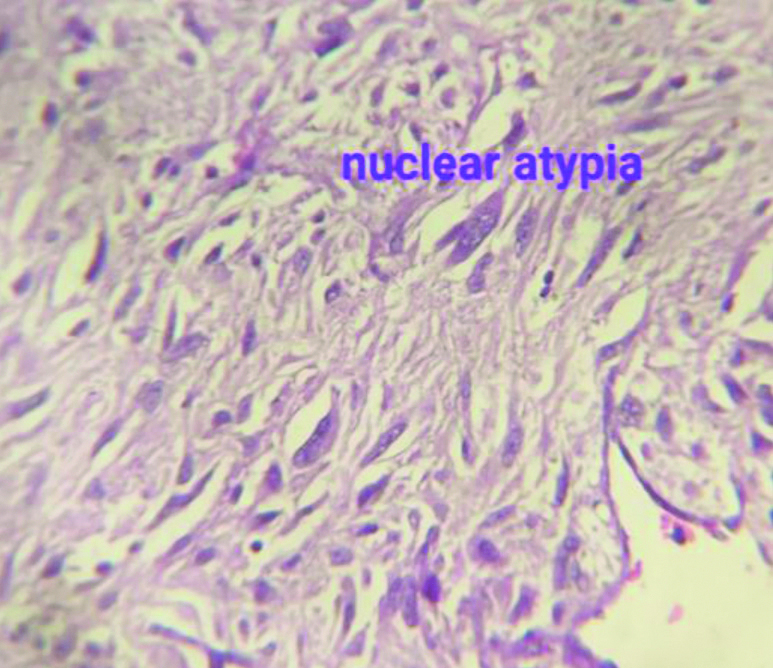
Histopathology (H&E stain) image in high power (400x magnification) field showing nuclear pleomorphism, atypia and high nuclear to cytoplasmic ratio.
Postoperative course was uneventful. Patient was discharged with feeding jejunostomy. On third week follow-up, feeding jejunostomy was removed. As histopathology revealed probable malignant potential with high risk for metastases, oncologist opinion was taken and patient was started with adjuvant Imatinib for 3 years. She had been advised for regular follow-up at 3 months, 6 months and one year.
Discussion
GISTs are rare tumours with an annual incidence of 10-15 per million [1]. However, they are the most common mesenchymal tumours of the Gastrointestinal Tract (GIT) and mostly arise from the stomach (60%), jejunum and ileum (30%) [2]. Duodenal GISTs constitute 30% of primary duodenal tumours and less than 5% of GISTs [3]. EGISTs arise from urinary bladder, gallbladder, omentum and mesentery and should be clearly separated from the GITs [4]. EGIST arising from the pancreas are very rare [5]. The most common location of the pancreatic GISTs was the head of pancreas, followed by the tail of pancreas [6].
GISTs originate from the interstitial cells of Cajal in the GIT. These cells are present in other organs too, hence GIST subsets may also exist [7]. They are usually asymptomatic [5]. In symptomatic patients, the clinical symptoms include abdominal pain, flatulence, vomiting, ileal bleeding, anaemia and weight loss. It can be diagnosed incidentally from radiologic imaging [8,9]. The patient mentioned, presented with pain and vomiting due to compression of duodenum by the mass.
The imaging findings including CT features of GISTs vary greatly. They primarily depend on the size and aggressiveness of the tumour and the time of presentation. Small GISTs are predominantly solid with homogeneous moderate enhancement. Large GISTs are typically hypervascular, enhancing masses with areas of heterogeneous appearance due of necrosis, haemorrhage or cystic degeneration. The findings in the above case were large mass having heterogenous enhancement in the arterial phase and necrosis. Ulceration and fistulisation to the gastrointestinal lumen are also common features. The tumours displace adjacent organs and may invade the structures in advanced cases, as in pancreatic invasion [10,11]. They can be non-specific in case of EGIST those arise from pancreas.
Because of the hypervascular nature and enlarged arterial and venous channels in this case, the imaging differential of non-functional NET was also considered. Being a young female patient and an area of haemorrhage with leveling, another differential of SPEN of the pancreas was kept.
Primary duodenal GISTs are generally large, well-defined, heterogeneously enhancing, hypervascular masses with a prominent mixed growth pattern on CT. Cai PQ et al., found duodenal GISTs with prominent blood supply having intratumoural vasculature and veins in their arterial phase [12].
Through Endoscopic Ultrasound-Fine Needle Aspiration (EUS-FNA) 38-89% of luminal GISTs can be diagnosed [13]. It is higher for pancreatic lesions and further better accuracy (75-96%) was reported for adenocarcinomas [14]. FNA or biopsy was not performed in this case to avoid the risk of tumour seeding and bleeding.
Microscopically, the tumour consists of a spindle cell type (60-70%) or an epithelioid type (20-30%) or as a mixture. GISTs typically express the c-KIT protein (CD 117). Other typical markers for GISTs are the antibody Discovered on GIST-1 (DOG-1), the cell surface glycoprotein CD34 (70%), and ASMA (40%). GISTs are mostly negative for desmin and S-100 [15-17]. In the above case, microscopy revealed tumour arising from the muscular layer of duodenum with infiltration into the pancreatic head. It consisted of ovoid and spindle shaped tumour cells with hyperchromatic nuclei, moderate pleomorphism and was positive for DOG1 and CD34.
The prognosis of patients with GIST depends on the biological behaviour and location. GISTs arising from the distal GIT tend to be more aggressive. According to Reith JD et al., nearly half of 48 patients with EGIST died of metastatic or recurrent tumours within an average of 2 years [4]. This reflects that, the prognosis of EGIST is similar to GIST arising in the distal GIT with slightly aggressive course than the GIST arising from stomach. Fletcher CD et al., and Miettinen M and Lasota J used tumour size and mitotic numbers to define the criteria for estimating the aggressive behaviour and metastatic risk of GIST [1,18]. GISTs are divided into very low (<2 cm, <5/50 HPF), low (2-5 cm, <5/50 HPF), intermediate (<10 cm, <5/50 HPF) and high risk metastases (>5 cm, >5/50 HPF or >10 cm, all mitotic numbers). Accordingly the patient was having high metastatic risk as the tumour size >10 cm and mitosis <5/50 HPF.
Management for GISTs include primary surgery, neoadjuvant chemotherapy followed by surgery, adjuvant therapy after surgery. Metastatic and advanced stages require debulking surgery. Resectable tumour are treated by surgery either by laparoscopy or laparotomy depending on size and location. More than 1 cm tumour free margin is considered as potentially curative [19,20]. In case of unresectable tumour or with metastatic spread, neoadjuvant chemotherapy with imatinib should be performed and surgery after 3-4 months re-evaluation is generally performed [21,22]. Duodenal GISTs frequently metastasize to the liver and the peritoneal cavity, including the omentum and the mesentery. Metastasis to the lymph nodes, bones, lungs, or subcutaneous tissues may occur rarely [23]. As lymph node metastasis is very rare; therefore, radical surgery is generally not needed [15].
Conclusion(s)
GISTs arising from duodenum are rare and mostly arise in the second part. Frequently, the mass is large making it difficult to distinguish from pancreatic head carcinoma or NET as observed in the given case. However, duodenal GIST can be differentiated from pancreatic head tumours because of its rich vascularity and early draining veins. Also, biliary obstruction is rare in duodenal GIST unlike pancreatic head tumours. As a radiologist, one should know all issues related to different imaging pattern.
[1]. Fletcher CD, Berman JJ, Corless C, Gorstein F, Lasota J, Longley BJ, Diagnosis of gastrointestinal stromal tumors: A consensus approachHum Pathol 2002 33(5):459-65.10.1053/hupa.2002.12354512094370 [Google Scholar] [CrossRef] [PubMed]
[2]. Miettinen M, Lasota J, Gastrointestinal stromal tumours- Definition, clinical, histological, immunohistochemical, and molecular genetic features and differential diagnosisVirchows Arch 2001 438(1):01-12.10.1007/s00428000033811213830 [Google Scholar] [CrossRef] [PubMed]
[3]. Miettinen M, Kopczynski J, Makhlouf HR, Sarlomo-Rikala M, Gyorffy H, Burke A, Gastrointestinal stromal tumors, intramural leiomyomas and leiomyosarcomas in the duodenum: A clinicopathologic, immunohistochemical and molecular genetic study of 167 casesAm J Surg Pathol 2003 27(5):625-41.10.1097/00000478-200305000-0000612717247 [Google Scholar] [CrossRef] [PubMed]
[4]. Reith JD, Goldblum JR, Lyles RH, Weiss SW, Extragastrointestinal (soft tissue) stromal tumours: An analysis of 48 cases with emphasis on histologic predictors of outcomeMod Pathol 2000 13(5):577-85.10.1038/modpathol.388009910824931 [Google Scholar] [CrossRef] [PubMed]
[5]. Akahoshi K, Sumida Y, Matsui N, Oya M, Akinaga R, Kubokawa M, Preoperative diagnosis of gastrointestinal stromal tumour by endoscopic ultrasound-guided fine needle aspirationWorld J Gastroenterol 2007 13(14):2077-82.10.3748/wjg.v13.i14.207717465451 [Google Scholar] [CrossRef] [PubMed]
[6]. Akbulut S, Yavuz R, Otan E, Hatipoglu S, Pancreatic extragastrointestinal stromal tumor: A case report and comprehensive literature reviewWorld J Gastrointest Surg 2014 6(9):175-82.10.4240/wjgs.v6.i9.17525276287 [Google Scholar] [CrossRef] [PubMed]
[7]. Miettinen M, Are desmoid tumors KIT positive?Am J Surg Pathol 2001 25(4):549-50.10.1097/00000478-200104000-0002811257638 [Google Scholar] [CrossRef] [PubMed]
[8]. Showalter SL, Lloyd JM, Glassman DT, Berger AC, Extra-gastrointestinal stromal tumor of the pancreas: Case report and a review of the literatureArchives of Surgery 2008 143:305-08.10.1001/archsurg.2007.6818347279 [Google Scholar] [CrossRef] [PubMed]
[9]. Shah S, Mortele KJ, Uncommon solid pancreatic neoplasms: Ultrasound, computed tomography, and magnetic resonance imaging featuresSeminars in Ultrasound, CT and MR 2007 28(5):357-70.10.1053/j.sult.2007.08.00217970552 [Google Scholar] [CrossRef] [PubMed]
[10]. Levy AD, Remotti HE, Thompson WM, Sobin LH, Miettinen M, Gastrointestinal stromal tumors: Radiologic features with pathologic correlationRadioGraphics 2003 23(2):283-304.10.1148/rg.23202514612640147 [Google Scholar] [CrossRef] [PubMed]
[11]. Burkill GJ, Badran M, Al-Muderis O, Thomas JM, Judson IR, Fisher C, Malignant gastrointestinal stromal tumor: Distribution, imaging features, and pattern of metastatic spreadRadiology 2003 226(2):527-32.10.1148/radiol.226201188012563150 [Google Scholar] [CrossRef] [PubMed]
[12]. Cai PQ, Lv XF, Tian L, Luo ZP, Mitteer RA Jr, Fan Y, CT characterization of duodenal gastrointestinal stromal tumorsAm J Roentgenology 2015 204(5):988-93.10.2214/AJR.14.1287025905932 [Google Scholar] [CrossRef] [PubMed]
[13]. Williams DB, Sahai AV, Aabakken L, Penman ID, van Velse A, Webb J, Endoscopic ultrasound guided fine needle aspiration biopsy: A large single centre experienceGut 1999 44(5):720-26.10.1136/gut.44.5.72010205212 [Google Scholar] [CrossRef] [PubMed]
[14]. Harewood GC, Wiersema MJ, Endosonography-guided fine needle aspiration biopsy in the evaluation of pancreatic massesAm J Gastroenterol 2002 97(6):1386-91.10.1111/j.1572-0241.2002.05777.x12094855 [Google Scholar] [CrossRef] [PubMed]
[15]. Miettinen M, Lasota J, Gastrointestinal stromal tumors: Review on morphology, molecular pathology, prognosis, and differential diagnosisArchives of Pathology and Laboratory Medicine 2006 130(10):1466-78.10.5858/2006-130-1466-GSTROM17090188 [Google Scholar] [CrossRef] [PubMed]
[16]. Beham AW, Schaefer IM, Schüler P, Cameron S, Ghadimi BM, Gastrointestinal stromal tumorsInternational Journal of Colorectal Disease 2012 27(6):689-700.10.1007/s00384-011-1353-y22124674 [Google Scholar] [CrossRef] [PubMed]
[17]. Tan CB, Zhi WQ, Shahzad G, Mustacchia P, Gastrointestinal stromal tumors: A review of case reports, diagnosis, treatment, and future directionsISRN Gastroenterology 2012 2012:59596810.5402/2012/59596822577569 [Google Scholar] [CrossRef] [PubMed]
[18]. Miettinen M, Lasota J, Gastrointestinal stromal tumors: Pathology and prognosis at different sitesSeminars in Diagnostic Pathology 2006 23(2):70-83.10.1053/j.semdp.2006.09.00117193820 [Google Scholar] [CrossRef] [PubMed]
[19]. Chaudhry UI, DeMatteo RP, Management of resectable gastrointestinal stromal tumorHematology/Oncology Clinics of North America 2009 23(1):79-87.10.1016/j.hoc.2009.01.00119248972 [Google Scholar] [CrossRef] [PubMed]
[20]. Langer C, Schüler P, Becker H, Liersch T, Gastrointestinal stromal tumors from the surgical point of viewLaparoscopic Therapy. Der Chirurg 2008 79(7):644-49.10.1007/s00104-008-1528-418496659 [Google Scholar] [CrossRef] [PubMed]
[21]. Gronchi A, Fiore M, Miselli F, Lagonigro MS, Coco P, Messina A, Surgery of residual disease following molecular-targeted therapy with imatinib mesylate in advanced/metastatic GISTAnnals of Surgery 2007 245(3):341-46.10.1097/01.sla.0000242710.36384.1b17435538 [Google Scholar] [CrossRef] [PubMed]
[22]. Raut P, Posner M, Desai J, Morgan JA, George S, Zahrieh D, Surgical management of advanced gastrointestinal stromal tumors after treatment with targeted systemic therapy using kinase inhibitorsJ Clin Oncol 2006 24(15):2325-31.10.1200/JCO.2005.05.343916710031 [Google Scholar] [CrossRef] [PubMed]
[23]. Cheng JM, Tirumani SH, Shinagare AB, Jagannathan JP, Hornick JL, Raut CP, MDCT of primary, locally recurrent, and metastatic duodenal gastrointestinal stromal tumours (GISTs): A single-institution study of 25 patients with review of literatureClin Radiol 2014 69(2):137-44.10.1016/j.crad.2013.09.00224161459 [Google Scholar] [CrossRef] [PubMed]