Breast cancer is a heterogeneous disease and has significant variability in presentation, treatment response, and prognosis. The management of carcinoma breast is currently based on immunophenotypes and Tumour Nodes Metastasis (TNM) staging. The aim of the study is to review the literature and to look for treatment guidelines of carcinoma breast, the role of immunophenotypes in treatment of carcinoma breast, TP53 mutation in breast cancer, and the relationship of TP53 mutation with immunophenotypes, histological grade, efficacy of chemotherapy, and BRCA (Breast Cancer gene) mutation. Pubmed database was researched and a total of 510 articles were analysed. A total of one meta-analysis, one randomised controlled trial, one literature review, nine prospective studies, and three retrospective studies were included for further analysis. It was observed that TP53 mutation is associated with poor overall survival. It was also found to be inversely associated with the Estrogen Receptor (ER) status and so it was seen more commonly in basal type and HER2/neu enriched breast cancer. There is a need for further studies to establish the definite association between TP53 and immunophenotypes so that TP53 alone can be used as a guide for management in carcinoma breast at low resource centres.
Introduction
Breast cancer is the most common cancer among women and also has greatest number of cancer-related deaths among women. The incidence of breast cancer is 2.1 million cases per year and there were 6,27,000 breast cancer related deaths in 2018, as per the World Health Organisation (WHO) [1]. The advent of screening guidelines leading to early diagnosis of cases of breast cancer along with the multimodality treatment with operative intervention, chemotherapy, radiation therapy, and hormonal therapy has resulted in a 5-year survival of more than 90% [2]. In spite of multimodality treatment, the cases of carcinoma breast show a variable response, with a subset of breast carcinoma cases not responding well to these and having a high incidence of recurrence, thereby effecting the disease-free survival and overall survival.
Breast cancer, as a result of the variability seen in presentation, treatment response and prognosis has long been understood as a heterogeneous disease and various efforts have been made to classify it further to guide the treatment. Initial research focussed on histopathological variants and around 20 major, as well as 18 minor subtypes of breast carcinoma has been described in the WHO classification [3]. The TNM staging, as defined by Pierre Denoix includes tumour size, lymph node involvement, and distant metastasis was widely used to plan management of the carcinoma breast cases. The treatment of carcinoma breast, for a considerable time, was guided by histological grading and TNM staging but it was realised that tumours with same TNM and histological grade were showing variable response and prognosis and hence search for alternate classification was still of significant interest. This led to the molecular classification as suggested by Perou CM and Sorlie T, in which carcinoma breast was divided into subgroups according to gene expressions, ‘luminal’ group reflecting the expression of ER genes, and the expression of genes expressed in normal luminal epithelial cells, ‘HER2 positive’ reflecting ErbB2/HER2 gene expression, ‘basal’ reflecting no expression of ER and HER2 genes, and the expression of genes expressed in breast basal and myoepithelial cells [4,5]. It was then realised that carcinoma breast can be subclassified into Luminal A and Luminal B, HER2 enriched and basal subtype based on genes expressing phenotypes of ER, Progesterone Receptor (PR), and HER2 instead of hundreds of intrinsic genes [6,7]. In 2013, the St. Gallen guidelines gave Immunohistochemistry (IHC) based molecular classification for clinical decision making, which is now the mainstay of planning treatment of the patient with carcinoma breast and also has been included in the TNM classification by AJCC 8th edition [8]. [Table/Fig-1] describes St. Gallen-Intrinsic subtypes of breast cancer [9].
St. Gallen-Intrinsic Subtypes of Breast Cancer [9].
| Intrinsic type | Clinicopathological definition |
|---|
| Luminal A | ER +PR high +HER2 −Low Ki-67 (<14%) |
| Luminal B | ER+PR low or intermediate+HER2+ or HER2−Ki-67 ≥14% |
| ErB-B2 overexpression | HER2 +ER −PR − |
| Basal Like | HER2 −ER −PR − |
ER: Estrogen receptor; PR: Progesterone receptor
Materials and Methods
The scientific literature was reviewed to look for current evidence related to treatment guidelines of carcinoma breast, the role of immunophenotypes in the treatment of carcinoma breast, TP53 mutation and its relationship with immunophenotypes and histological grade. The database used was Pubmed and the MeSH used were carcinoma breast, immunophenotypes, estrogen receptor, progesterone receptor, TP53/p53, triple negative breast cancer, and histological grade.
A total of 510 articles were analysed using the above-stated MeSH terms and those articles studying the clinicopathological profile of TP53 in breast cancer and the relationship of TP53 with immunophenotypes and histological grade were included for analysis. Those studies that dealt with male breast cancer, were animal studies, case series, or small retrospective studies were excluded. These studies were analysed for the sample size studied, the period of follow-up, and the outcome. Univariate and multivariate analysis was used by most studies to establish the role of TP53 in carcinoma breast and the meta-analysis had used the funnel plot.
Immunophenotypes and Histological Grading
The immunophenotypes were found to be significantly related to the histopathological grading as seen in the study by Shukla S et al., Luminal A and Luminal B cancers were most commonly seen to have low-grade carcinoma breast, whereas triple-negative breast cancer and Her2/neu enriched breast cancer has a higher histological grade [10]. This association between immunophenotypes and histological grade has led to histological grading being used as an adjunct in the management of carcinoma breast.
TP53
TP53, also known as the guardian of the genome, is a tumour suppressor gene, located in Chromosome 17p13. The p53 protein coded by the TP53 gene is located in the nucleus of cells throughout the body. In normal cells, TP53 expression is low due to MDM2 [11]. After the damage to the DNA in a cell by toxic chemicals, radiation, or Ultraviolet (UV) rays from sunlight, activation of Ataxia Telangiectasia Mutated (ATM) and ATR (Ataxia Telangiectasia and Rad3 Related) protein kinase leads to phosphorylation and activation of TP53 and increases the level of p53 protein. The activated TP53 functions by three mechanisms which are repair of the DNA, growth arrest, or apoptosis.
If the DNA can be repaired, TP53 activates genes like P53R2 to fix the damage. The cells in which damaged DNA cannot be repaired are directed towards apoptosis, as proliferation of cells with damaged DNA can lead to carcinoma [12]. The pathway for apoptosis involves BAX (Bcl 2 associated X protein), which stimulates cytochrome c and apoptosis is induced by binding of cytochrome c to caspase 9 [13].
TP53 gene can also cause the arrest of the cell cycle at major cell cycle checkpoints. Cell cycle arrest is mediated by p21 gene. The expression of p21 leads to inhibition of CDK complex and phosphorylation of RB (Retinoblastoma) gene. Inhibition of RB prevents cells from entering the G1 phase and inhibition of CDK 2 prevent cells from entering into the S phase [14]. This arrest can be temporary and gives the cell time to repair the DNA or permanent and irreversible leading to senescence.
TP53 mutation is found in approximately 50% of human cancers. The study of TP53 mutation in 33 common cancers revealed that half of the cancers have TP53 mutation in more than 50% cases, whereas, two-thirds of cancers have TP53 mutation in more than 30% of patients [15]. Almost 20-40% of carcinoma breast cases are associated with TP53 mutation [16]. TP53 mutation in carcinoma breast can be inherited or non-inherited. The inherited mutation is part of Li-Fraumeni syndrome and accounts for a small part of breast carcinoma cases. Non-inherited mutation, which forms the majority of breast carcinoma cases are acquired during a person’s life and is usually a change in single amino acid of the protein leading to loss of tumour suppressor function of the TP53 gene. Mutated TP53 does not bind to MDM2, accumulates in the nucleus, and can be detected by immunochemistry along with other methods like cDNA sequencing and luminometric immunoassay [17]. [Table/Fig-2] shows the location of the TP53 gene [12]. [Table/Fig-3,4] shows the immunostaining of TP53.
Cytogenetic Location: 17p13.1, which is the short (p) arm of chromosome 17, at position 13.1 [12].
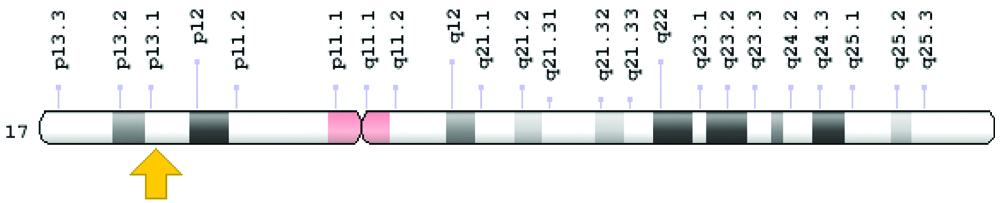
TP53 positive immunostaining. Malignant cells with brown colour nuclear staining for p53 (X400).
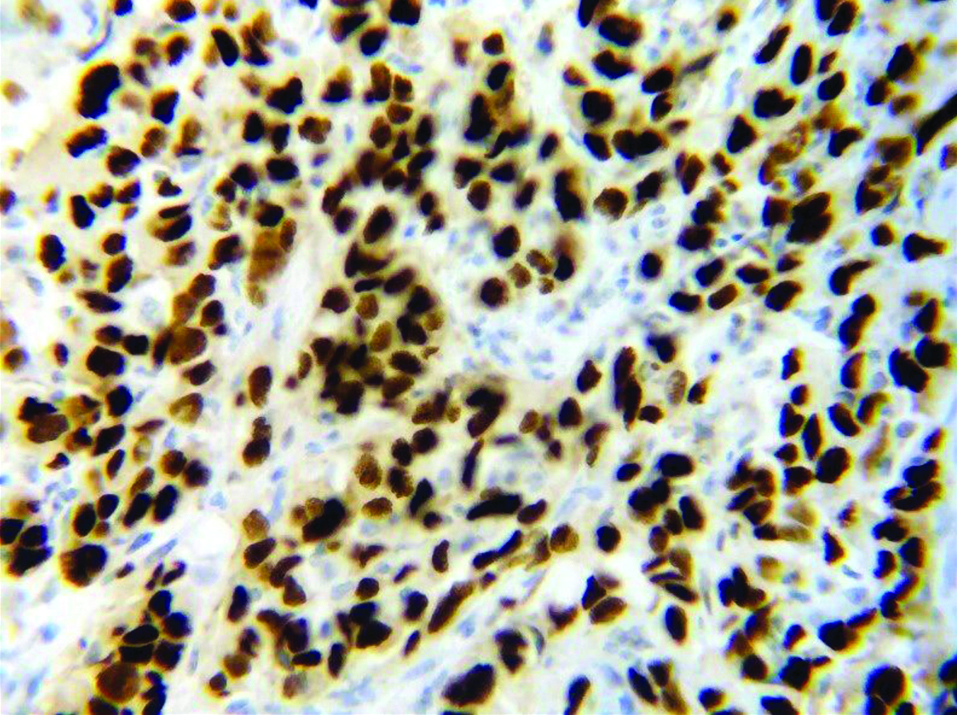
TP53 negative immunostaining. No brown colour nuclear staining for p53 (X400).
TP53 and Carcinoma Breast
The research for the role of TP53 in breast cancer had started in the early 1990s and the one of the first meta-analysis by Barbareschi M which included more than 9000 patients, did not reveal a strong correlation between TP53 and prognosis of breast cancer [18]. The TP53 mutation detection in the initial studies was mostly based on immunohistochemistry but with the advent of DNA sequencing, it was employed by most of the studies which led to a higher yield of TP53 mutations. The literature review by Hartmann A et al., Blaszyk H et al., and Pharoah PDP et al., together studied around 6000 patients from 25 studies and a strong prognostic significance of TP53 mutations in carcinoma breast was reported [19-21]. Most of the studies had a drawback of being retrospective and were difficult to compare due to the use of different variables. Pharoah PDP et al., published a meta-analysis using 16 studies, which were compatible, with 3500 patients and the funnel plot revealed a significant association between TP53 mutation and overall survival [21].
Iacopetta B et al., who studied 422 patients and followed-up for 72 months, concluded that TP53 mutation is associated with poor overall survival as well as disease-free survival [22]. Barnes DM et al., studied 195 cases with a follow-up of 10 years and had similar results [23]. Berns EM et al., studied 222 patients and concluded that TP53 mutation is associated with poor disease-free survival [24]. Andersen TI et al., also, with a sample size of 179, showed a statistically significant association between TP53 mutation and disease-free as well as overall survival [25]. Seshadri R et al., with 919 cases and follow-up period of 66 months showed TP53 mutation was associated with increased relapse and death in breast carcinoma [26]. Another prospective study by Falette N et al., in which 113 patients were assessed for TP53 mutation using DNA sequencing and were followed-up for 105 months, revealed that TP53 mutation was an independent prognostic marker of early relapse and death [27]. These studies were part of the meta-analysis.
Blaszyk H et al., in a prospective study with 90 patients who were followed-up for a period of 60 months revealed that TP53 mutation as the single most adverse prognostic indicator for recurrence and death as well as for response to adjuvant and palliative treatment [20]. Another prospective study by Mac Grogan G et al., in which 942 patients were assessed for TP53 mutation using DNA sequencing and were followed-up for 117 months, revealed that TP53 mutation was an independent prognostic marker in carcinoma breast [28]. Jung SY et al., studied 845 patients with carcinoma breast for TP53 mutation and immunophenotypes, for a mean duration of 66 months and concluded that TP53 mutation gives an additional prognostic significance for immunophenotypes [29]. Malamou-Mitsi V et al., in a study where 595 patients were randomised for dose-dense chemotherapy also showed that TP53 mutation on these was associated with poor histological grade, high recurrence, and death. The study also showed an association between TP53 mutation and ER-negative status [30]. González JD et al., with his study published in 2017, had followed-up 102 patients for a period of 26 months and showed that TP53 mutation is associated with lower survival [31]. [Table/Fig-5] provides the list of studies that establishes the role of TP53 as a prognostic indicator for carcinoma breast [20,21,28-31].
Studies regarding TP53 as an prognostic indicator for carcinoma breast [20,21,28-31].
| Study (Year of publication) | Type of study | Patients | Follow-up (Months) | Outcome |
|---|
| Mac Grogan G et al., [28] (1995) | Prospective | 942 | 117 | TP53 as an independent prognostic marker |
| Pharoah PDP et al., [21] (1999) | Meta-analysis | 3500 | - | TP53 mutation associated with poor overall survival |
| Blaszyk H et al., [20] (2000) | Prospective | 90 | 60 | TP53 indicator of recurrence and death |
| Malamou-Mitsi V et al., [30] (2006) | Prospective | 595 | 50 | TP53 associated with high histological grade, high recurrence and death |
| Jung SY et al., [29] (2011) | Prospective | 845 | 66 | TP53 additional prognostic significance for immunophentype |
| González JD et al., [31] (2017) | Prospective | 102 | 26 | TP53 associated with lower survival |
TP53 and BRCA (Breast Cancer Gene)
BRCA-1 gene, which is also a tumour suppressor gene, is responsible for almost 50% of inherited breast cancer. BRCA-1 is similar to TP53 in terms of activation via phosphorylation as well as the downstream pathways involved in functioning of BRCA and TP53. In spite of these similarities, Arizti P et al., in the study of the correlation between TP53 and BRCA showed that stress that leads to DNA damage and TP53 stimulation down-regulates BRCA, and also cell cycle arrest and senescence by TP53 contributes to down-regulation of BRCA. They also demonstrated that BRCA-1 expression depends on presence of wild type TP53 [32].
TP53 and Chemotherapy
The study of the impact of TP53 mutation on response to chemotherapy has shown conflicting results. Tiezzi DG et al., in a study with 60 patients who were administered preoperative docetaxel and epirubicin showed that immunochemical markers like TP53 expression did not predict response to chemotherapy [33]. Similarly, Bonnefoi H et al., also, in a randomised controlled trial between taxane and non-taxane based chemotherapy, showed that TP53 status did not predict the sensitivity to taxane-based chemotherapy [34]. However, Sakuma K et al., have shown that TP53 over-expression in triple-negative breast cancer is associated with better response to anthracyclin and taxane-based neoadjuvant chemotherapy [35]. This conflicting result has been explained by Bertheau P et al., due to the pro-apoptotic nature of TP53 that induces tumour cell death in response to chemotherapy-induced DNA damages, and on the other hand, it induces cell cycle arrest, protecting tumour cells from damage from chemotherapy drugs, especially for high-dose and dose-intense epirubicin-cyclophosphamide regimen [36].
TP53 and Histological Grade
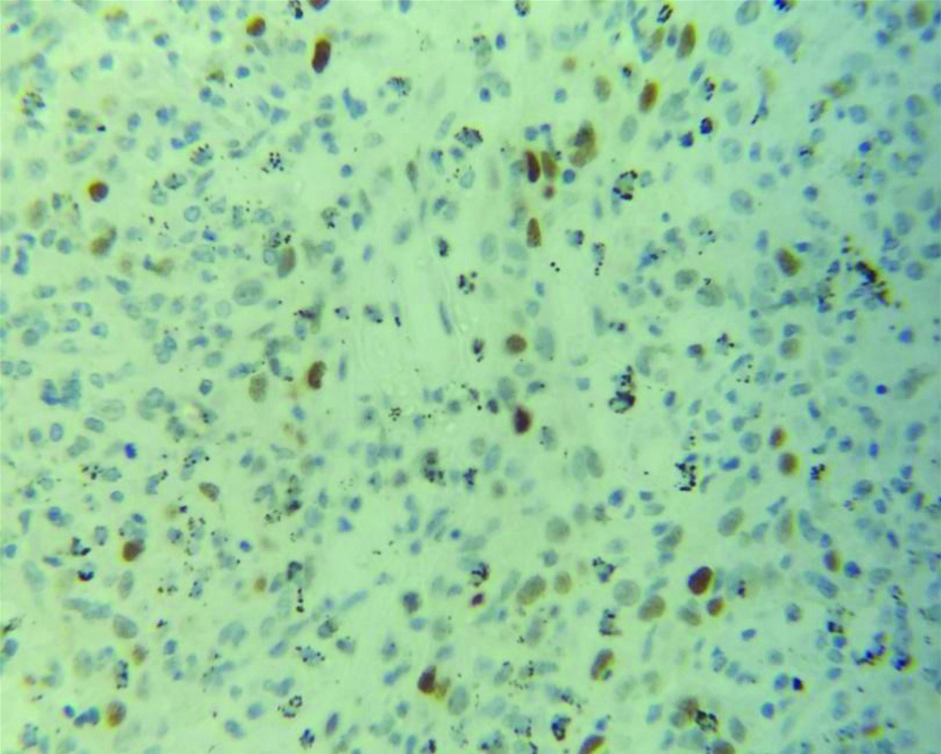
The histological grading of carcinoma breast has been known to determine the prognosis on cases of carcinoma breast. The Nottingham modification of Bloom Richardson grading is based on tumour tubule formation, the number of mitotic figures in most active areas, and nuclear pleomorphism. Each of the criteria is awarded 1-3 points. The tumour is graded as well-differentiated (3-5 points=Grade 1), moderately differentiated (6-7 points=Grade 2) and poorly differentiated (8-9 points=Grade 3) based on representative portion and not the least differentiated portion. [Table/Fig-6,7] shows low grade and high grade carcinoma breast. The study of the clinicopathological profile of carcinoma breast with TP53 mutation also revealed that tumour with TP53 mutation is associated with a higher histological grade which has been seen in the studies by Anderson TI et al., Mac Grogan G et al., and Malamou-Mitsi V et al., [25,28,30].
Section showing histopathological features suggestive of Infiltrating Ductal Carcinoma (NOS type) of BR grade 1 (H&E X100).
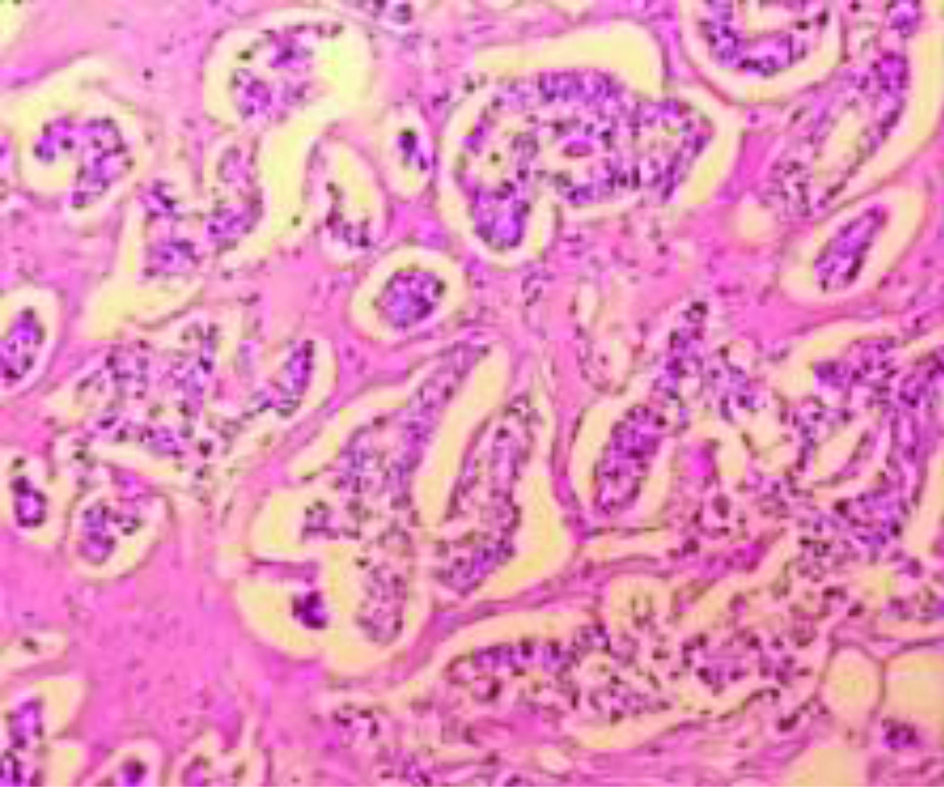
Section showing histopathological features suggestive of Infiltrating Ductal Carcinoma (NOS type) of BR grade 3 (H&E X100). High Grade (BR Grade 3) Carcinoma Breast.
TP53 and Immunophenotypes
In an attempt to establish TP53 as an independent prognostic marker which can be used to modify the treatment of patients with carcinoma breast, it was also established that TP53 mutations are present significantly higher in basal subtype whereas lower in Luminal subtypes. Tsutsui S et al., in a prospective study with 514 patients with a median follow-up of 30 months found that TP53 and ER status are independent factors for prognosis and also there was an inverse correlation between ER receptor status and TP53 mutation [37]. These inferences are similar to those by Malamou-Mitsi V et al., [30]. Coates AS et al., in the study on 1113 patients concluded that TP53 positivity was present more commonly in patients with ER-negative tumours, and also TP53 status in ER-negative tumours was associated with better disease-free survival [38]. Putti TC et al., also studied 291 ER-negative tumours and showed higher TP53 expression [39]. Kim JY et al., studied 174 cases of breast cancer and found increased TP53 mutation in triple-negative breast cancer [40]. Atik E et al., studied various immunohistochemical markers in 36 triple-negative and 15 non-triple-negative breast cancer and showed a higher level of TP53 expression in triple-negative breast cancer [41].
Conclusion(s)
The understanding of TP53 mutation in breast carcinoma is essential, the direct correlation with higher histological grade and, triple-negative and HER2 enriched immunophenotypes, opens up new opportunities for research, to establish the relation between TP53 and immunophenotypes in a better way, so definite guidelines can be laid down for carcinoma breast management. The current guidelines for the management of breast carcinoma involving immunophenotypes are not available in resource-poor rural setups hence there is a need to look for an alternate variables which correlate well with the current immunophenotypes and at the same time are easily accessible in primary and secondary care centres. TP53 is one such marker, whose expression, when measured by immunohistochemistry can be utilised in resource-poor settings as it will be cost-effective. The current literature does not give enough evidence to use TP53 alone as a variable to guide management of carcinoma breast. Therefore, there is a need to initiate new studies aiming to establish TP53 as a marker to guide the management of carcinoma breast.
ER: Estrogen receptor; PR: Progesterone receptor
[1]. WHO | Breast cancer. WHO. http://www.who.int/cancer/prevention/diagnosis-screening/breast-cancer/en [Google Scholar]
[2]. Cancer of the Breast (Female)- Cancer Stat Facts. SEER. https://seer.cancer.gov/statfacts/html/breast.html [Google Scholar]
[3]. Ellis IO et al. (2003) in World Health Organisation Classification of Tumours. Pathology and Genetics of Tumours of the Breast and Female Genital Organs, eds Tavassoli FA, Devilee P (IARC Press, Lyon, France), pp. 13-59, 277-279, 322-323 [Google Scholar]
[4]. Perou CM, Sørlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, Molecular portraits of human breast tumoursNature 2000 406(6797):747-52.10.1038/3502109310963602 [Google Scholar] [CrossRef] [PubMed]
[5]. Sørlie T, Perou CM, Tibshirani R, Aas T, Geisler S, Johnsen H, Gene expression patterns of breast carcinomas distinguish tumour subclasses with clinical implicationsProc Natl Acad Sci USA 2001 98(19):10869-74.10.1073/pnas.19136709811553815 [Google Scholar] [CrossRef] [PubMed]
[6]. Fragomeni SM, Sciallis A, Jeruss JS, Molecular subtypes and local-regional control of breast cancerSurg Oncol Clin N Am 2018 27(1):95-120.10.1016/j.soc.2017.08.00529132568 [Google Scholar] [CrossRef] [PubMed]
[7]. Kapp AV, Jeffrey SS, Langerød A, Børresen-Dale AL, Han W, Noh DY, Discovery and validation of breast cancer subtypesBMC Genomics 2006 7:23110.1186/1471-2164-7-23116965636 [Google Scholar] [CrossRef] [PubMed]
[8]. Goldhirsch A, Wood WC, Coates AS, Gelber RD, Thürlimann B, Senn H-J, Strategies for subtypes-dealing with the diversity of breast cancer: Highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011Ann Oncol Off J Eur Soc Med Oncol 2011 22(8):1736-47.10.1093/annonc/mdr30421709140 [Google Scholar] [CrossRef] [PubMed]
[9]. Coates AS, Winer EP, Goldhirsch A, Gelber RD, Gnant M, Piccart-Gebhart M, Tailoring therapies-improving the management of early breast cancer: St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2015Ann Oncol Off J Eur Soc Med Oncol 2015 26(8):1533-46.10.1093/annonc/mdv22125939896 [Google Scholar] [CrossRef] [PubMed]
[10]. Shukla S, Acharya S, Vagha S, Dawande P, Tamhane A, Role of immunophenotypes in carcinoma breastInt J Appl Basic Med Res 2018 8(4):210-16. [Google Scholar]
[11]. Momand J, Zambetti GP, Olson DC, George D, Levine AJ, The mdm-2 oncogene product forms a complex with the p53 protein and inhibits p53-mediated transactivationCell 1992 69(7):1237-45.10.1016/0092-8674(92)90644-R [Google Scholar] [CrossRef]
[12]. GH. TP53 gene [Internet]. Genetics Home Reference. [cited 2019 Dec 5]. Available from: https://ghr.nlm.nih.gov/gene/TP53 [Google Scholar]
[13]. Miyashita T, Reed JC, Tumour suppressor p53 is a direct transcriptional activator of the human bax geneCell 1995 80(2):293-99.10.1016/0092-8674(95)90412-3 [Google Scholar] [CrossRef]
[14]. Mendoza-Rodríguez CA, Cerbón MA, Tumour suppressor gene p53: Mechanisms of action in cell proliferation and deathRev Investig Clin Organo Hosp Enfermedades Nutr 2001 53(3):266-73. [Google Scholar]
[15]. Wang X, Sun Q, TP53 mutations, expression and interaction networks in human cancersOncotarget 2016 8(1):624-43.10.18632/oncotarget.1348327880943 [Google Scholar] [CrossRef] [PubMed]
[16]. Børresen-Dale AL, TP53 and breast cancerHum Mutat 2003 21(3):292-300.10.1002/humu.1017412619115 [Google Scholar] [CrossRef] [PubMed]
[17]. Norberg T, Lennerstrand J, Inganäs M, Bergh J, Comparison between p53 protein measurements using the luminometric immunoassay and immunohistochemistry with detection of p53 gene mutations using cDNA sequencing in human breast tumoursInt J Cancer 1998 79(4):376-83.10.1002/(SICI)1097-0215(19980821)79:4<376::AID-IJC12>3.0.CO;2-3 [Google Scholar] [CrossRef]
[18]. Barbareschi M, Prognostic value of the immunohistochemical expression of p53 in breast carcinomas: A review of the literature involving over 9,000 patientsAppl Immunohistochem 1996 4(2):106-16. [Google Scholar]
[19]. Hartmann A, Blaszyk H, Kovach JS, Sommer SS, The molecular epidemiology of P53 gene mutations in human breast cancerTrends Genet 1997 13(1):27-33.10.1016/S0168-9525(96)10043-3 [Google Scholar] [CrossRef]
[20]. Blaszyk H, Hartmann A, Cunningham JM, Schaid D, Wold LE, Kovach JS, A prospective trial of midwest breast cancer patients: A p53 gene mutation is the most important predictor of adverse outcomeInt J Cancer 2000 89(1):32-38.10.1002/(SICI)1097-0215(20000120)89:1<32::AID-IJC6>3.0.CO;2-G [Google Scholar] [CrossRef]
[21]. Pharoah PDP, Day NE, Caldas C, Somatic mutations in the p53 gene and prognosis in breast cancer: A meta-analysisBr J Cancer 1999 80(12):1968-73.10.1038/sj.bjc.669062810471047 [Google Scholar] [CrossRef] [PubMed]
[22]. Iacopetta B, Grieu F, Powell B, Soong R, McCaul K, Seshadri R, Analysis of p53 gene mutation by polymerase chain reaction-single strand conformation polymorphism provides independent prognostic information in node-negative breast cancerClin Cancer Res Off J Am Assoc Cancer Res 1998 4(7):1597-602. [Google Scholar]
[23]. Barnes DM, Dublin EA, Fisher CJ, Levison DA, Millis RR, Immunohistochemical detection of p53 protein in mammary carcinoma: An important new independent indicator of prognosis?Hum Pathol 1993 24(5):469-76.10.1016/0046-8177(93)90158-D [Google Scholar] [CrossRef]
[24]. Berns EM, van Staveren IL, Look MP, Smid M, Klijn JG, Foekens JA, Mutations in residues of TP53 that directly contact DNA predict poor outcome in human primary breast cancerBr J Cancer 1998 77(7):1130-36.10.1038/bjc.1998.1879569050 [Google Scholar] [CrossRef] [PubMed]
[25]. Andersen TI, Holm R, Nesland JM, Heimdal KR, Ottestad L, Børresen AL, Prognostic significance of TP53 alterations in breast carcinomaBr J Cancer 1993 68(3):540-48.10.1038/bjc.1993.383 [Google Scholar] [CrossRef]
[26]. Seshadri R, Leong AS, McCaul K, Firgaira FA, Setlur V, Horsfall DJ, Relationship between p53 gene abnormalities and other tumour characteristics in breast-cancer prognosisInt J Cancer 1996 69(2):135-41.10.1002/(SICI)1097-0215(19960422)69:2<135::AID-IJC12>3.0.CO;2-8 [Google Scholar] [CrossRef]
[27]. Falette N, Paperin MP, Treilleux I, Gratadour AC, Peloux N, Mignotte H, Prognostic value of P53 gene mutations in a large series of node-negative breast cancer patientsCancer Res 1998 58(7):1451-55. [Google Scholar]
[28]. MacGrogan G, Bonichon F, de Mascarel I, Trojani M, Durand M, Avril A, Prognostic value of p53 in breast invasive ductal carcinoma: An immunohistochemical study on 942 casesBreast Cancer Res Treat 1995 36(1):71-81.10.1007/BF006901877579509 [Google Scholar] [CrossRef] [PubMed]
[29]. Jung SY, Jeong J, Shin SH, Kwon Y, Kim EA, Ko KL, Accumulation of p53 determined by immunohistochemistry as a prognostic marker in node negative breast cancer; analysis according to st gallen consensus and intrinsic subtypesJ Surg Oncol 2011 103(3):207-11.10.1002/jso.2181921337548 [Google Scholar] [CrossRef] [PubMed]
[30]. Malamou-Mitsi V, Gogas H, Dafni U, Bourli A, Fillipidis T, Sotiropoulou M, Evaluation of the prognostic and predictive value of p53 and Bcl-2 in breast cancer patients participating in a randomised study with dose-dense sequential adjuvant chemotherapyAnn Oncol Off J Eur Soc Med Oncol 2006 17(10):1504-11.10.1093/annonc/mdl14716968874 [Google Scholar] [CrossRef] [PubMed]
[31]. González JD, Lagunas VM, González JD, Lopez-Arellano ME, Muñoz-Camacho J, Teran-Porcayo MA, Overexpression of p53 protein is a marker of poor prognosis in Mexican women with breast cancerOncol Rep 2017 37(5):3026-36.10.3892/or.2017.555328393224 [Google Scholar] [CrossRef] [PubMed]
[32]. Arizti P, Fang L, Park I, Yin Y, Solomon E, Ouchi T, Tumour suppressor p53 is required to modulate BRCA1 expressionMol Cell Biol 2000 20(20):7450-59.10.1128/MCB.20.20.7450-7459.200011003642 [Google Scholar] [CrossRef] [PubMed]
[33]. Tiezzi DG, Andrade JM, Ribeiro-Silva A, Zola FE, Marana HRC, Tiezzi MG, HER-2, p53, p21 and hormonal receptors proteins expression as predictive factors of response and prognosis in locally advanced breast cancer treated with neoadjuvant docetaxel plus epirubicin combinationBMC Cancer 2007 7:3610.1186/1471-2407-7-3617324279 [Google Scholar] [CrossRef] [PubMed]
[34]. Bonnefoi H, Piccart M, Bogaerts J, Mauriac L, Fumoleau P, Brain E, TP53 status for prediction of sensitivity to taxane versus non-taxane neoadjuvant chemotherapy in breast cancer (EORTC 10994/BIG 1-00): A randomised phase 3 trialLancet Oncol 2011 12(6):527-39.10.1016/S1470-2045(11)70094-8 [Google Scholar] [CrossRef]
[35]. Sakuma K, Kurosumi M, Oba H, Kobayashi Y, Takei H, Inoue K, Pathological tumour response to neoadjuvant chemotherapy using anthracycline and taxanes in patients with triple-negative breast cancerExp Ther Med 2011 2(2):257-64.10.3892/etm.2011.21222977494 [Google Scholar] [CrossRef] [PubMed]
[36]. Bertheau P, Espié M, Turpin E, Lehmann J, Plassa LF, Varna M, TP53 status and response to chemotherapy in breast cancerPathobiology 2008 75(2):132-39.10.1159/00012385118544968 [Google Scholar] [CrossRef] [PubMed]
[37]. Tsutsui S, Ohno S, Murakam S, Hachitanda Y, Oda S, Prognostic value of p53 protein expression in breast cancer; an immunohistochemical analysis of frozen sections in 514 Japanese womenBreast Cancer Tokyo Jpn 2001 8(3):194-201.10.1007/BF0296750811668240 [Google Scholar] [CrossRef] [PubMed]
[38]. Coates AS, Millar EK, O’Toole SA, Molloy TJ, Viale G, Goldhirsch A, Prognostic interaction between expression of p53 and estrogen receptor in patients with node-negative breast cancer: Results from IBCSG Trials VIII and IXBreast Cancer Res BCR 2012 14(6):R14310.1186/bcr334823127292 [Google Scholar] [CrossRef] [PubMed]
[39]. Putti TC, El-Rehim DMA, Rakha EA, Paish CE, Lee AHS, Pinder SE, Estrogen receptor-negative breast carcinomas: A review of morphology and immunophenotypical analysisMod Pathol Off J U S Can Acad Pathol Inc 2005 18(1):26-35.10.1038/modpathol.380025515332092 [Google Scholar] [CrossRef] [PubMed]
[40]. Kim JY, Park K, Jung HH, Lee E, Cho EY, Lee KH, Association between mutation and expression of TP53 as a potential prognostic marker of triple-negative breast cancerCancer Res Treat Off J Korean Cancer Assoc 2016 48(4):1338-50.10.4143/crt.2015.43026910472 [Google Scholar] [CrossRef] [PubMed]
[41]. Atik E, Guray M, Ozgur T, Canda T, Characterization of immunohistochemical markers in triple negative breast carcinomasJBUON 2013 18(4):886-90. [Google Scholar]