The Acute Febrile Illness (AFI) is a common cause of patients’ visit to hospital in India. AFI poses diagnostic and therapeutic challenge, particularly in resource constrained settings. Scrub typhus is an important cause of AFI in India and outbreaks of scrub typhus have been reported from various states [1-4]. In Karnataka, scrub typhus has been studied in few areas, taking into account mainly the clinical features and serology {Weil-Felix and IgM Enzyme Linked Immunosorbent Assay (ELISA)} but there is scarcity of comprehensive studies with confirmed diagnosis (by MIF or molecular methods) and follow-up of patients to know the outcome [5]. Scrub typhus, a re-emerging infectious disease is caused by O. tsutsugamushi and transmitted through the bite of larval forms (chiggers) of trombiculid mites (predominantly Leptotrombidium deliense) [5,6]. Though considered as disease of rural areas, this disease has become urbanised and the occurrence has broadened.
In India, scrub typhus is usually under diagnosed due to poor suspicion by the clinicians and lack of access to appropriate diagnostic tests in most places [7]. Delay in diagnosis and treatment can prove fatal, with case fatality as high as 30% [8]. Early diagnosis is the key and once diagnosed, treatment with doxycycline is affordable and mostly successful with dramatic clinical response within 48 hours, which can be taken as therapeutic test. Azithromycin and chloramphenicol are other antibiotics useful for treatment [3]. However, reports of emergence of antibiotic-resistant strains of O. tsutsugamushi may be a cause of concern and at present there are no vaccines available against scrub typhus [9,10]. Clinical spectrum of scrub typhus may vary from subclinical to clinically overt presentation (fever, myalgia, headache, abdominal pain, lymphadenopathy, hepatosplenomegaly, eschar etc.,) to multiorgan failure and death in untreated cases [11]. Eschar at the site of bite by larval mite, though pathognomonic of scrub typhus, may not be seen in all cases [4]. So practically, it’s a challenge for clinician to differentiate scrub typhus from other febrile illnesses. Laboratory aid thus becomes imperative and can be done either by serological tests or by molecular methods [12].
Weil-Felix test is the oldest and most commonly used test in resource constrained settings even today, in spite of limited sensitivity and specificity [13]. Other serological tests are rapid card tests, ELISA, MIF, immunoperoxidase assay etc. ELISA is practically very useful, though MIF is considered as gold standard [14]. However, serological methods are limited by their capacity for early detection due to low level of serum antibodies during early course of disease. Molecular methods like PCR are therefore said to be useful for early and confirmed diagnosis [15]. In addition, PCR-RFLP is also useful for strain typing [13,16]. Several antigenic strain types of O. tsutsugamushi have been recognised, main serotypes being Gilliam, Karp, Boryong and Kato. The antigenic strain diversity is attributable to high variation in the 56-kDa type-specific antigen [17]. Earlier, serologic testing (viz., complement fixation, immunofluorescence) based on 56 kDa protein, was used to identify strain types. Later RFLP analysis was employed, which also showed a high degree of variability between the digestion patterns of isolates [18]. However, RFLP and immunofluorescence techniques have shown discrepancies indicating high degree of genetic diversity among O. tsutsugamushi isolates. Genotyping, based on 56-kDa gene for phylogenetic analyses in comparison with prototype and reference strains is currently being used to identify strain types [19].
Given this background, there is need to comprehensively study the clinical spectrum, outcome of patients and laboratory diagnosis by different methods viz., serology (Weil-Felix, ELISA and MIF) and molecular method. Also, strain typing in scrub typhus is essential. There is paucity of studies encompassing all these aspects, especially from Karnataka, India. Present study on scrub typhus was taken up at Bengaluru, sought to prospectively study the cases, document the outcome, comprehensively diagnose scrub typhus by serology and molecular method and perform strain typing (MIF, PCR-RFLP and phylogeny), as well as follow-up cases to demonstrate fourfold rise in antibody titre by MIF.
Materials and Methods
Prospective cross-sectional study was conducted in a Tertiary Care Hospital in Bengaluru, Southern India for two years (June 2015-May 2017). Institutional Ethical Clearance (BMCRI/PS/415/2014-15) and written informed consent from the patients were obtained. One hundred consecutive patients presenting with AFI where scrub typhus (rickettsiosis) was clinically suspected and other common causes of fever were ruled out during the study period, were enrolled and were categorised as clinically suspected cases, confirmed cases and probable cases of scrub typhus (as per DHR-ICMR guidelines for diagnosis and management of Rickettsial diseases in India 2015) [20]. Detailed clinical history was taken; signs and symptoms were recorded in the proforma. Patients diagnosed with febrile illness other than scrub typhus viz., dengue, malaria, pneumonia, leptospirosis and typhoid by clinicians were excluded from the study. Results of laboratory investigations were recorded from medical records wherever feasible.
Serum samples and EDTA blood (3 mL each) were collected from the patients for diagnosis of scrub typhus. Serum samples were analysed for scrub typhus by Weil-Felix test, IgM ELISA, MIF IgM and IgG and EDTA blood for PCR-RFLP. Weil Felix test was performed using commercially available kit (Omega Diagnostics Ltd., Scotland, UK). Titre of ≥1: 80 for OX K were taken as significant for scrub typhus. Scrub Typhus Detect IgM ELISA (InBios International Inc., Seattle, WA) was performed, following the manufacturer’s instructions. Optical density of >0.5 was considered positive [20]. MIF IgM and IgG for scrub typhus was performed with commercially available kit (Fuller Laboratories, CA, USA) as per manufacturer’s instructions. For IgM, titre of >1:64 and for IgG, >1: 128, was taken as positive [21]. Serum samples with significant titres for MIF IgG were further processed to obtain end point titre. All the patients were followed-up; convalescent serum samples were collected at the end of four weeks from those who gave consent. Samples were tested for demonstration of four-fold rise in antibody titre by MIF IgG [21].
For PCR-RFLP, bacterial DNA (Deoxyribonucleic acid) was extracted from EDTA blood, using a QIAamp DNA Mini Kit (Qiagen, Hilden, Germany). Nested PCR targeting the 56-kDa protein of O.tsutsugamushi was carried out on all 100 samples as reported previously [16]. Briefly, PCR was performed with nested primer pairs 34 (5’-TCAAGCTTATTGCTAGTGCAATGTCTGC-3’) and 55 (5’-GGGATCCCTGCTGCTGTGCTTGCTGCG-3’) for first PCR cycle and 10 (5-’GATCAAGCTlTCCTCAGCCTACTATAATGCC-3’) and 11 (5’-CTAGGGATCCCGACAGATGCACTATTAGGC-3’) for the second PCR cycle [16]. The PCR amplification mixture (total volume of 50 μL) contained PCR master mix including Taq polymerase and template DNA. The mixture was denatured at 94°C for 30 seconds and annealed at 57°C for 2 minutes. The chain was extended at 70°C for 2 minutes in thermocycler for 30 cycles. Amplicons were electrophorosed using 1.5% agarose gel, stained with ethidium bromide and observed under Ultra violet transilluminator. Known positive DNA of O.tsutusgamushi was used as positive control. The sample was considered positive when 487 bp specific band was detected. Strain typing was performed by MIF, RFLP and sequencing and phylogeny. For sequencing and phylogeny, 21 selected amplicons (487 bp) obtained by PCR were utilised (data already published) [22]. MIF strain typing was attempted with the same commercially available kit (Fuller Laboratories, CA, USA) used for MIF IgM test. Each MIF slide contains 10 wells. Every well contains four separately prespotted strains (Kato, Karp, Gilliam and Boryong) of O. tsutsugamushi, with each strain forming a micro dot that is separated from the others. On addition of serum, it allows the detection of antibodies in the serum samples against all four individual antigens simultaneously [23]. Spot showing maximum fluorescence in a well, was taken as strain type. The slide was read by two observers independently to overcome subjectivity. For RFLP, 10 μL of amplified PCR product was digested with 10 U of restriction endonuclease Hae III in 15 μL of reaction volume and incubated at 37°C for 6 hours. The digested and undigested bands were analysed after electrophoresis in 1.5% agarose gel, stained with dye and observed under Ultra violet transilluminator [15].
Statistical Analysis
Data was entered in Microsoft excel spreadsheet. The basic demographic data was summarised using descriptive statistics. Data was analysed using SPSS software.
Results
Demographic and Epidemiological Profile
Patients in the study belonged to urban and rural areas in and around Bengaluru. A 52% were from Bengaluru city, 22% from Tumkur, 15% from Chikballapura, 11% from Ramnagara and other surrounding areas [Table/Fig-1].
Map showing cases of scrub typhus from areas in and around Bangalore, Karnataka, India.
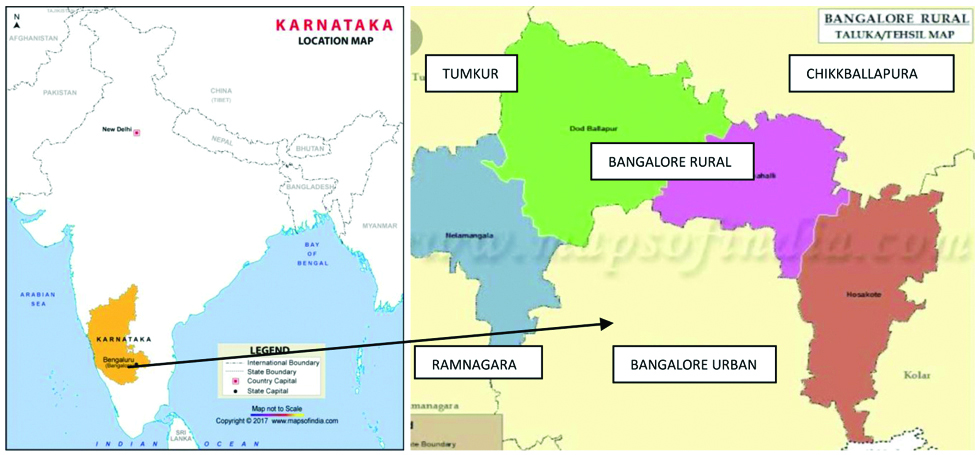
Seasonal trend was evident, majority of the cases presented during monsoon and winter seasons with peak in July, August and November. Most of the patients (76%) belonged to paediatric age group, mean age of adults was 32.9 years and that of paediatric cases was 8.7 years; slight male preponderance was seen (55%) [Table/Fig-2].
Clinical and laboratory profile of cases.
| Patient characteristics | N=100 |
|---|
| General features |
| Paediatric | 76 |
| Adult | 24 |
| Gender |
| Male | 55 |
| Female | 45 |
| Seasonality |
| Winter | 53 |
| Monsoon | 41 |
| Summer | 06 |
| Clinical symptoms |
| Fever | 100 |
| Rash | 27 |
| Vomiting | 19 |
| Headache | 18 |
| Pain abdomen | 17 |
| Altered sensorium | 12 |
| Joint pain | 09 |
| Pedal oedema | 05 |
| Clinical signs |
| Rash | 32 |
| Pallor | 16 |
| Icterous | 08 |
| Eschar | 04 |
| Hepatosplenomegaly | 53 |
| CNS involvement | 39 |
| Pleural effusion | 08 |
| Laboratory findings |
| Thrombocytopenia | 52 |
| Elevated liver enzymes | 24 |
| Anaemia | 15 |
| Abnormal WBC count | 11 |
Clinical and Laboratory Profile
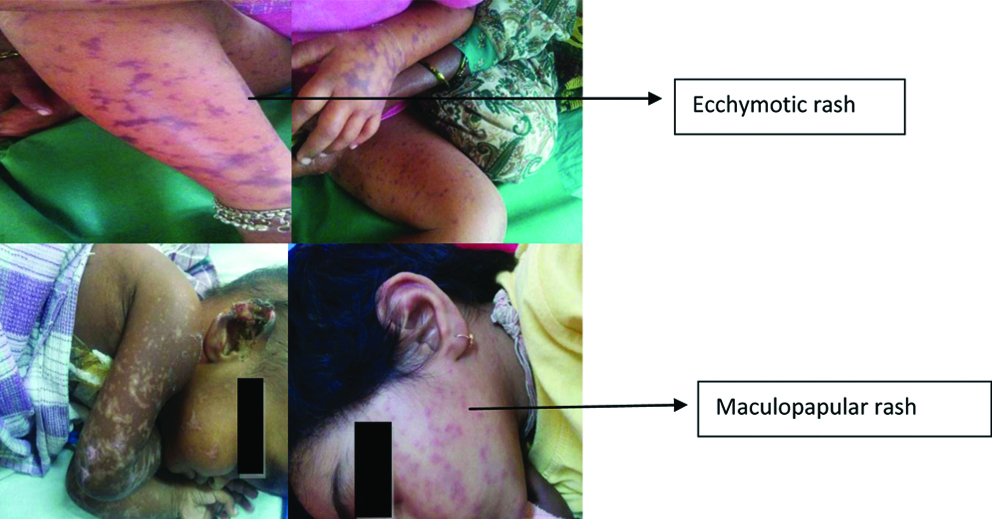
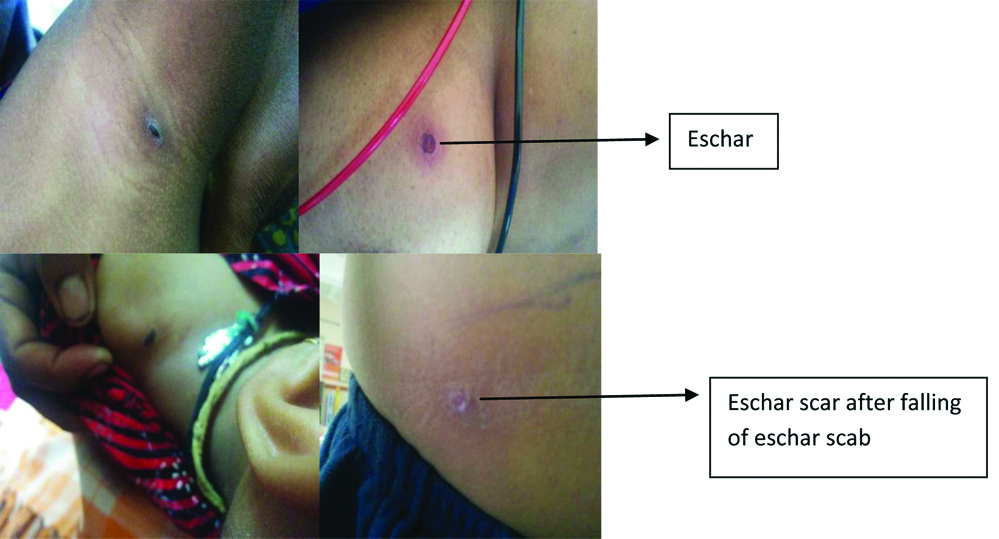
All the patients had fever with average duration being eight days. The other common symptoms were rash (27%), vomiting (19%), headache (18%), pain abdomen (17%), altered sensorium (12%), joint pain (9%) and pedal oedema (5%). On general physical examination, rash [Table/Fig-3], was seen in 32%, pallor in 16%, icterous in 08% and eschar, which is diagnostic of scrub typhus in 04% of patients (even after thorough examination in all cases), including one scar where eschar scab had fallen-off [Table/Fig-4]. Systemic examination revealed hepatosplenomegaly in 53%, signs of CNS involvement in 39% and pleural effusion in 08% patients. Laboratory investigations is shown in [Table/Fig-2]. Among all laboratory parameters, percentage of abnormal LFT was found to be higher (46%) in patients infected with Gilliam strain, however it was not found to be statistically significant.
Rash (maculopapular/ecchymotic) seen in cases of scrub typhus.
Eschar and eschar scab seen in cases of scrub typhus.
Laboratory Profile for Diagnosis Scrub Typhus [
Table/Fig-5]
Laboratory profile for diagnosis scrub typhus.
| Investigation | Positive/No tested (n) | Percentage |
|---|
| Weil felix test (OX K >80) | 45/100 | 45% |
| IgM ELISA* | 26/90 | 28.89% |
| MIF Ig M | 34/100 | 34% |
| MIF-Ig G | 19/100 | 19% |
| NESTED PCR | 22/100 | 22% |
*IgM ELISA was performed in only 90 patients, due to shortage of serum sample in 10 patients
A 45% of the cases showed a titer >80 for OX K by Weil Felix test, with 8% showing titre of >1280. A total of 28.89% were positive for IgM ELISA. MIF IgM [Table/Fig-6], was positive in 34 patients and MIF IgG in 19 cases. Titres for MIF IgG ranged from 1:128 to 1:1024. Nested PCR for 56kDa was positive in 22 cases. A total of 25 patients gave consent to provide follow-up samples at the end of four weeks from the time of 1st sample collection. MIF IgG was performed on these 25 convalescent samples, of which samples from 10 patients showed fourfold rise in antibody titre. Out of these 10 patients, PCR was negative in four patients.
MicroImmunoFluorescence (MIF): Negative and Positive results in MIF.
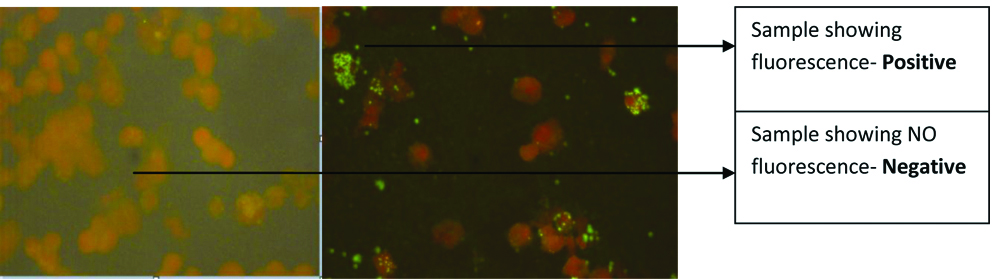
As per DHR-ICMR guidelines for diagnosis and management of Rickettsial diseases in India 2015, all 100 patients included in study were clinically suspected cases, 26% confirmed cases (22 were PCR positive and additional 04 patients, with negative PCR showed four-fold rise in antibody titre by MIF IgG) and 12% of them were probable cases (only OX K titre of ≥1:80 by Weil Felix test and OD value >0.5 in ELISA). In all, there were 100 clinically suspected cases and 38 laboratory diagnosed (confirmed and probable) cases of scrub typhus. MIF strain typing showed presence of all the major strain types of O.tsutsugamushi, with predominance of Karp strain (44%) followed by Gilliam (21%) and rest 11% were Boryong and 9% were Kato and 15% strains were untypable. PCR-RFLP (PCR restriction analysis) was performed for 22 PCR positive cases, which showed 3 RFLP patterns [Table/Fig-7].
RFLP showing 3 patterns (single digestion band at 200 bp, two digestion bands at 174 bp and 313 bp and band with no restriction site at 487 bp).
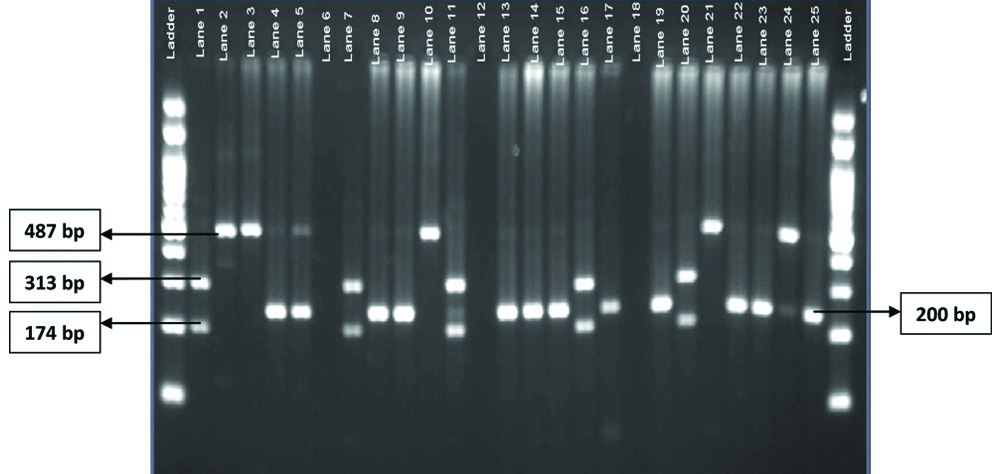
RFLP results correlated well with phylogeny results, 12 (54%) had RFLP profile with single digestion band at 200 bp and corresponded with Gilliam strains, 05 that showed two digestion bands at 174 bp and 313 bp and another 05 that had no restriction site in RFLP showed corresponding results in phylogeny.
Follow-up and outcome [Table/Fig-8]: As per DHR-ICMR guidelines, patients were treated with doxycycline, which is the drug of choice (200 mg in two divided doses for adults and 4.5 mg/kg bodyweight for children for 7 days), majority of the patients responded to treatment with doxycycline and recovered, except for 12% who succumbed and 2% were lost for follow-up.
| Parameter | N=100 | Percentage |
|---|
| Treatment with doxycycline | 100 | 100 |
| Improved outcome | 86 | 86 |
| Death | 12 | 12 |
| Lost for follow-up | 02 | 02 |
Discussion
Scrub typhus has reemerged as an important and treatable cause of AFI in our country [23]. Laboratory evidence of scrub typhus was found in 38% of patients in this study. Majority of this patients were from urban and rural areas in and around Bengaluru, Karnataka, India. Though most of the studies have reported rural preponderance [24,25], presence of scrub typhus in urban areas cannot be ruled out, as noted in the present study. Seasonal surge observed in present study has been reported earlier as well [3,6,26]. This may be due to exposure to the bites of larval mites during the months of August to October which coincides with harvesting season in the fields. Further, growth of secondary scrub vegetation, which is the habitat for trombiculid mites in the immediate post-monsoon period, tends to contribute to the seasonal surge [6]. Paediatric preponderance was noted in this study, many other studies have been conducted on paediatric population in India and scrub typhus is found to be common among them [5,8,24]. Age is known to influence the occurrence of scrub typhus further epidemiological studies are required to substantiate. Symptoms of scrub typhus in this study were found to be non-specific in nature, like fever, rash, headache, nausea, vomiting, and pain abdomen and do not show much variation among subjects in different studies [6,8]. This reemphasises the need for high index of suspicion of scrub typhus in AFI. However, the presence of the eschar which is one of the important clinical manifestations of scrub typhus was seen only in 4% of the cases, similar finding has been reported by another study [22]. An eschar may be a highly variable finding, may be missed unless thoroughly searched for [27].
Common clinical findings encountered in the study were hepatosplenomegaly, CNS involvement and pleural effusion, similar to the findings in other studies. Gurunathan PS et al., reported hepatosplenomegaly in 80.6%, pleural effusion and pneumonitis in 26.9%, CNS involvement in 18.5% of cases and Narvencar CKP et al., in their study showed that 60% of cases had hepatomegaly and respiratory involvement [6,8]. Laboratory parameters, viz., abnormal leucocyte count, thrombocytopenia, abnormal LFT, abnormal RFT in this study were akin to many other studies [3,6,25]. Clinical manifestations and severity of the symptoms are reported to vary with different genotypes of O. tsutsugamushi [1,28]. No such symptomatic variation was found in the present study, however percentage of abnormal LFT was found to be higher (46%) in the patients infected with Gilliam strain. Variations in laboratory findings with the genotypes of O. tsutsugamushi have been reported in a study by Anitha PK et al., as well, which showed that, strains closely aligned with Karp prototype were associated with elevated serum transaminases [28].
The mainstay of diagnosis in scrub typhus is serology [27]. The cheapest and widely used test in present country is Weil-Felix test. Serological evidence of scrub typhus was found in 45% of cases by Weil Felix test and in 28.89% of cases by IgM ELISA in our study, other studies have shown 77-96% positivity for Weil-Felix test with titer of 1:160 and 34% positivity by IgM ELISA [3,7,27]. The gold standard serological test i.e., four-fold rise in antibody titer by IgG MIF was seen in 10 (40%) of the patients, a study from Korea reported rise in 93% of their patients [29]. Higher rise seen in Korean study was due to inclusion of only confirmed cases for follow-up testing. However, interpretation of the four fold rise in antibody titre has to be done cautiously, as timing of collection of paired sera has a bearing on antibody titres and cut-off value selected for MIF may influence the diagnostic sensitivity. IgG has been reported to peak at four weeks and remain sustained for a longer period in comparison to IgM. PCR positivity in this study was 22%. Majority of the studies on antigenic heterogeneity of O. tsutsugamushi, have been done either using serological methods or by RFLP and recently by phylogenetic analysis [15,29]. In the present study, MIF strain typing showed predominance of Karp strains and did not match with sequencing and phylogeny results (data published elsewhere) [22]. Similar finding has been noted in other study as well. Study by Liu YX et al., showed that by IFA, 21 of the 23 isolates belonged to the Gilliam type, and two to the Karp type, where as RFLP analysis, showed that 21 strains had restriction profiles similar to the Japan Kawasaki strain and other two strains had restriction profiles similar to Karp and it was difficult to accurately identify the type of O. tsutsugamushi by serological methods [30]. Precise strain typing by MIF may be difficult due to the cross homology among antigens of various strains of O.tsutsugamushi. MIF though a gold standard technique for diagnosis, has limitations with regard to strain typing.
On the contrary, RFLP outcome correlated well with sequencing and phylogeny results (data published elsewhere) [22]. RFLP revealed three different patterns, similar to findings in study by Manosroi J et al., who showed that RFLP profiles were identical to the prototype O.tsutsugamushi [15]. The RFLP can be a useful preliminary tool for strain typing of O.tsutsugamushi, where sequencing and phylogeny is not possible. There was no geographical clustering of patients; various strain types were isolated from patients belonging to different locations in and around Bengaluru, Karnataka. Also, there was no association of clinical features with strain types reported in the present study. Majority of the patients responded to treatment with doxycycline, except for 12 who had complications like CNS involvement, septic shock, multiorgan failure etc., and succumbed. Scrub typhus is known to produce serious complications with mortality rate of 7-30% [27]. Deaths are attributable to late presentation, delayed diagnosis, presence of complications and criticality at admission and drug resistance.
Limitation(s)
Present study was conducted on patients presenting to Tertiary Care Centre where patients are referred from peripheral primary health centers or district hospitals for complications, hence it may not represent true burden of scrub typhus in the community. IgM ELISA was performed in only 90 patients, due to shortage of serum sample in 10 patients.
Conclusion(s)
Study showed that scrub typhus, should be considered in the differential diagnosis of AFI. Doxycycline may be used in empirical treatment of AFI where scrub typhus is suspected. Patients should be thoroughly examined for the presence of eschar/eschar scar, as eschar alone is sufficient to clinically distinguish scrub typhus from other causes of AFI. Serological cut-offs (MIF/ELISA) and PCR are practically more feasible for early confirmation, as follow-up testing for demonstration of four-fold rise in antibody titer may not always be possible. Different strain types exist in Southern India and PCR-RFLP can be a useful preliminary tool for strain typing, where sequencing and phylogeny is not possible. Early diagnosis by appropriate modality helps in improved outcome.