Comparative Study of FOXC1 Expression in Selective Odontogenic Cysts and Tumours
Thanit Prasitsak1, Chaidan Intapa2
1 Lecturer, Department of Oral Biology, Faculty of Dentistry, Naresuan University, Mueang, Phitsanulok, Thailand.
2 Assistant Professor, Department of Oral Diagnosis, Faculty of Dentistry, Naresuan University, Mueang, Phitsanulok, Thailand.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Thanit Prasitsak, Department of Oral Biology, Faculty of Dentistry, Naresuan University, Mueang, Phitsanulok, Thailand.
E-mail: thanitp@nu.ac.th
Introduction
Many studies have indicated that Forkhead Box C1 (FOXC1) is highly expressed in a various malignant neoplasms and its over expression is associated with tumour development, progression and metastasis. However, the role of FOXC1 in odontogenic lesions remains to be elucidated.
Aim
This study aimed to investigate FOXC1 expression in selective Odontogenic Cysts (OC) and Odontogenic Tumours (OT).
Materials and Methods
A descriptive study on OC and OT was performed on oral biopsy specimens at Faculty of Dentistry, Naresuan University, Phitsanulok, Thailand, between January 2009 and December 2016. Institute of Cancer Research (ICR) mouse were used as study animal. Immunohistochemical reaction was performed using antibody against FOXC1 in 23 formalin-fixed and paraffin-embedded tissues of eight Ameloblastoma (AM), one Adenomatoid Odontogenic Tumour (AOT), seven Odontogenic Keratocyst (OKC), five Dentigerous Cyst (DC), two Calcifying Odontogenic Cyst (COC) and three mouse embryonic head at Embryonic Day (E)14. The expression level of FOXC1 was evaluated using semi-quantitative analysis.
Results
Immunoreactivity of FOXC1 was not detected in the epithelial lining of OKC, DC and COC but it was identified in the epithelial compartment of AM and AOT. Overall, semi-quantitative analysis demonstrated moderate staining of FOXC1 and staining score was 4.13 in AM and 5 in AOT. In addition, FOXC1 was not detected in normal tooth bud of mouse including both enamel organ and condensing ectomesenchyme.
Conclusion
Expression of FOXC1 may contribute in tumouriogenesis of OT whereas it is may not be related with normal odontogenesis.
Embryonic head, Odontogenesis, Tooth bud, Tumouriogenesis
Introduction
Odontogenic Cysts (OC) and Odontogenic Tumours (OT) are common disorders of the jaw. Their aetiology and pathogenesis are related to genetic alteration of tooth forming cells [1,2]. Oncogenic signaling in OC and OT usually associated with normal odontogenic signaling pathways such as Sonic Hedgehog (SHH) signaling pathway, Wnt signaling pathway and Fibroblast Growth Factor (FGF) signaling pathway [1,3]. However, its mutation might be specific to OT but not in OC such as BRAFV600E mutations have been reported in AM but variably present in OKC [4-6]. Thus, a cluster of oncogenic genes in OC and OT remains to be elucidated to gain more knowledge in their pathogenesis.
The FOXC1 gene is located on chromosome 6p25 which function as a transcription factor that control normal biological function and related to tumouriogenesis [7,8]. During embryogenesis, FOXC1 is expressed in ectoderm, mesoderm and ectomesenchyme in head and neck region and play an important role in craniofacial development [7,9,10]. Overexpression of FOXC1 is related to cell proliferation, invasion and metastasis in a various type of cancers including tongue cancer and oral squamous cell carcinoma [11-15]. Knockdown of FOXC1 leads to inhibition of cell proliferation and migration in oral squamous cell carcinoma cell line [11]. Silencing of FOXC1 expression suppress epithelial-mesenchymal transition with enhanced cell invasion and migration in Tongue Squamous Cell Carcinomas (TSCC) cell line [12]. Thus, FOXC1 might functions as an oncogene in head and neck region and possibly related to OC and OT. There is no study about FOXC1 expression in odontogenic lesions till date.
This study aimed to investigate the expression of FOXC1 in selective OC and OT such as AM, AOT, DC, COC, OKC by immunohistochemistry (IHC) and compared its expression with normal tooth bud to gain knowledge of the possible role of FOXC1 in their pathogenesis.
Materials and Methods
Human Sample Selection
A descriptive study was performed on oral biopsy specimens from Faculty of Dentistry, Naresuan University, Phitsanulok, Thailand. Total 23 cases were obtained from the entire archive (January 2009 and December 2016) which were kept in a good condition. These were diagnosed by an experienced oral pathologist based on the World Health Organisation classification of head and neck tumours in 2017 [16]. Eight cases AM, one case AOT, seven cases OKC, five cases DC and two cases COC were included and analysed FOXC1 antibody. Protocol related to human specimen was conducted with the approval of the Naresuan University Institutional Review Board according to the Declaration of Helsinki (Approval No. 301/2016).
Animal
Institute of Cancer Research (ICR) mouse was purchased from National Laboratory Animal Center (Nakhon Pathom, Thailand). The morning on which a vaginal plug was identified was defined as embryonic day (E) 0. Embryonic head (n=3) was collected on the designated day (E14), then fixed in 4% Paraformaldehyde (PFA) in Phosphate-Buffered Saline (PBS) and processed for paraffin-embedded tissue. Animal protocol was approved by the Animal Welfare Committee of Center for Animal Research Naresuan University (Approval No. 5902008).
Immunohistochemistry and Haematoxylin Eosin Staining
Sections were cut at 5 μm in thickness, deparaffinised and rehydrated with series of decreasing alcohols. Antigen retrieval was performed in citrate buffer solution, then endogenous peroxidase activity was blocked with 3% H2O2 in methanol. Sections were incubated with 1.5% rabbit normal serum (PK 6105, Vectastain®) and followed with goat anti-FOXC1 (1:500, Abcam). Biotinylated rabbit anti-goat IgG was used as a secondary antibody and then incubated with avidin-biotin complex reagent (PK 6105, Vectastain®). Signal detection was performed by 3,3’-diaminobenzidine (DAB, Sigma). Counter staining was carried out in a 1% methyl green solution. Mouse embryo specimen was used as positive control. Specimen which was incubated in buffer without primary antibody was used as negative control. Haematoxylin eosin (H&E) staining was performed to confirm diagnosis. Image was captured by Olympus BX50 microscopy and DP73 digital camera (Olympus, Japan) with micro-imaging software (Olympus cellSens Dimension, version 1.6). A semi-quantitative analysis of FOXC1 staining was performed by using a method was modified from previously described method in literature [17].
Statistical Analysis
The collected data was subjected to descriptive statistical analysis.
Results
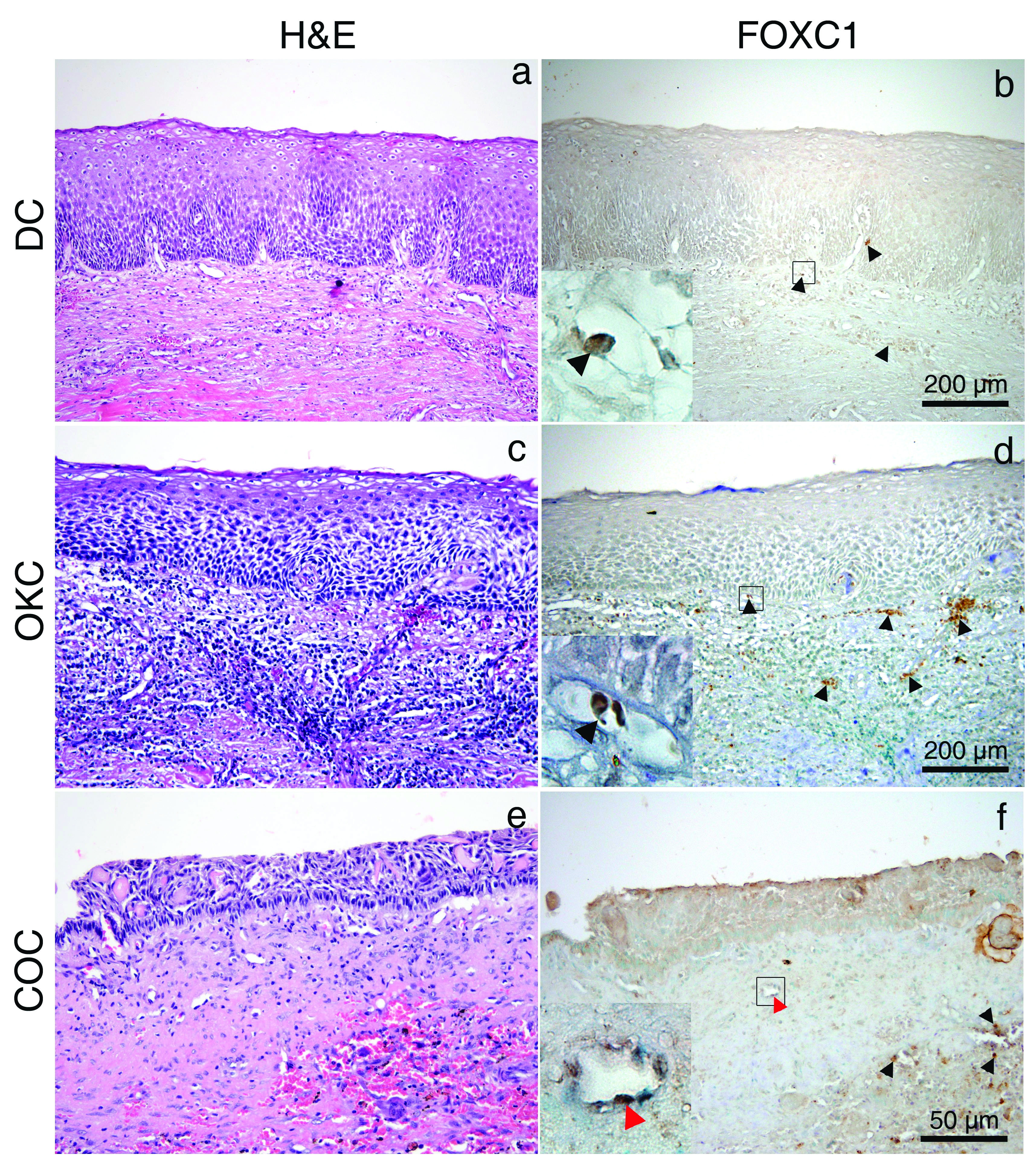
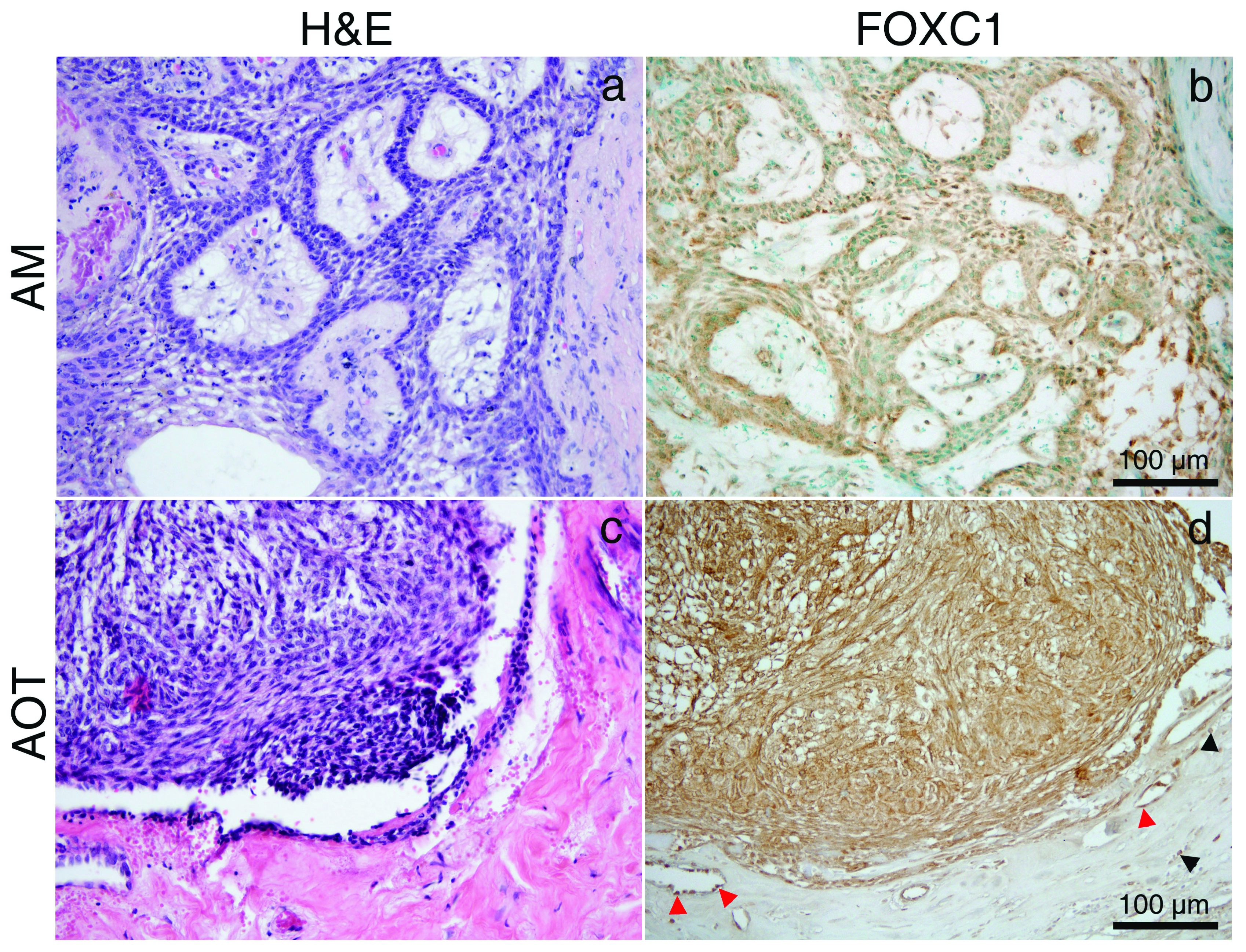
All specimens were stained with H&E to confirm diagnosis. DC, COC and OKC were used as representative of OC. IHC detection of FOXC1 in DC, OKC and COC was not detected in the epithelial lining of these OC [Table/Fig-1a-f]. AM and AOT were analysed as representative of OT from epithelial origin. Expression of FOXC1 was detected in the stellate reticulum-like cells and peripheral columnar ameloblast–like cells in the AM and spindle shaped and whorled mass of epithelial cells in the AOT [Table/Fig-2a-d]. Endothelial cell in the fibrous stromal part was detected in all specimens. There was no FOXC1 detection in the fibrous stromal part of AM and fibrous capsule of AOT similar to DC, COC and OKC. However, infiltrated red blood cells represented brown spot due to endogenous peroxidase activity in most samples. Semi-quantitative analysis of FOXC1 staining indicated that FOXC1 was expressed in all 8-cases of AM (weak expression in 25%; moderate expression in 50%; strong expression in 25%). The overall staining score was 4.13. AOT represented as a moderate expression of FOXC1 (staining score was 5).
H&E and Immunohistochemistry (IHC) of FOXC1 of DC (a,b), OKC (c,d), and COC (e,f). No expression of FOXC1 in the epithelial lining of OC; Magnified images of the boxed areas represent red blood cell (black arrow head) and endothelial cell (red arrow head). H&E and IHC staining; original magnification: ×100 (a-d), ×200 (e,f) and ×1000 (magnified images in boxed area).
H&E of AM (a); AOT (c). immunohistochemistry (IHC) of FOXC1 (brown stain) in stellate reticulum–like cells and peripheral columnar ameloblast-like cells in the AM; (b) and spindle shaped and whorled mass of epithelial cell in the AOT (d); red blood cells (black arrow head), endothelial cell (red arrow head). H&E and IHC staining; original magnification: ×200 (a-d).
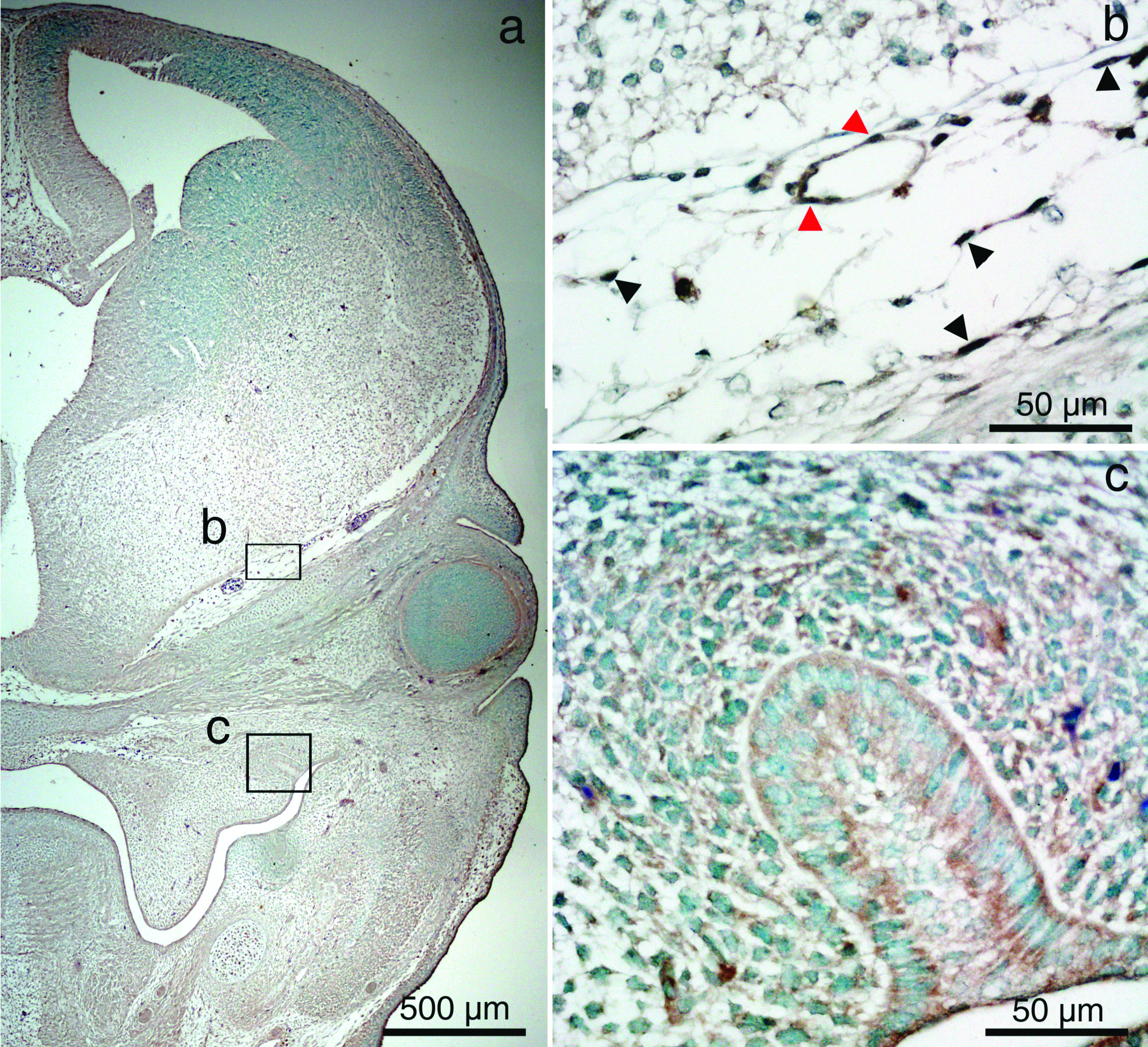
We investigated that FOXC1 expression in tooth bud of mouse and it relation to OC and OT cell origin. Embryonic heads at E14 were cut in coronal plane and detected FOXC1 protein localisation by IHC. FOXC1 protein localised to endothelial cells of perineural vascular plexus and head mesenchyme surround brain but there was no FOXC1 protein localisation to enamel organ and surrounding ectomesenchyme [Table/Fig-3].
Immunohistochemistry (IHC) of FOXC1 in mouse embryonic head on the coronal section at E14 (a-c). Perineural vascular plexus (b) and tooth bud (c) are magnified images of the boxed areas in (a). FOXC1 positive endothelial cells (red arrow heads) and head mesenchyme (black arrow heads) are detected but no FOXC1 positive cell in enamel organ and ectomesenchyme. IHC staining; original magnification: ×40 (a) and ×400 (b,c).
Discussion
FOXC1 is a transcription factor that control normal biological processes [7,10,18,19]. Abnormal regulation of FOXC1 gene in both loss and over function causes hereditary diseases and cancer such as Axenfeld–Rieger syndrome, Dandy-Walker malformation and oral squamous cell carcinoma [11,12,18-20]. During developmental process of mouse embryo, FOXC1 gene expressed in oral ectoderm and cranial ectomesenchyme at E8.5 and its activity was decreased and no longer detectable in the oral ectoderm. FOXC1 expression confines to ectomesenchyme of numerous structures in the head region such as the meninges, nasal septum, submandibular duct and gland at later stage [9,21]. Dental regulatory genes have been reported on OC and OT and there was no report about FOXC1 in dental producing cells [1,2]. Thus, this study investigated FOXC1 expression on tooth bud formation. This study found FOXC1 localisation on endothelial cells and cranial mesenchyme which was comparable to previous study but, it was not detected in enamel organ and condensing ectomesenchyme of embryonic mouse specimen [7]. However, it was reported that FOXC1 over expression inhibit odontogenic differentiation of dental pulp stem cells in vitro [22]. Thus, we suggest that FOXC1 gene functions in oral epithelium during early development and its role is ceased and could not detect its expression at morphogenesis stage of tooth development.
Overexpression of FOXC1 participates in oral cancers including TSCC with lymph node metastasis and oral squamous cell carcinoma. Oral squamous cell carcinoma cell line was decreased in cell proliferation and migration after FOXC1 suppression [11,13]. FOXC1 was also clearly detected in TSCC with lymph node metastasis compared to TSCC without lymph node metastasis [12]. Thus, it may participate in progression and aggressive behaviour in oral cancers. This study used AM and OKC to represent odontogenic lesions with aggressive behaviour and AOT, DC and COC to represent odontogenic lesions with nonaggressive behaviour but it revealed that FOXC1 was detected specifically in OT in both aggressive and nonaggressive behaviour lesions. Therefore, in odontogenic lesion, the function of FOXC1 might be different from those in oral cancer. Together with FOXC1 expression data in tooth bud, alteration of dental regulatory gene may not play a major role in a progression of OT. On the other hand, it could be suggested that FOXC1 might be an important transcription factor involved in pathogenesis and/or progression of OT and not associated with OC.
In addition, we used OKC sample, appropriate classification of which is still controversial. OKC was re-classified as a neoplastic lesion in 2005 and has been reinstated as an OC in 2017 due to mutation of PTCH1 gene, which was also detected in other developmental cyst and its clinical behaviour [16,23]. This study presented that FOXC1 was not observed in OKC and other OC. This evidence supports the classification of OKC as an OC.
However, with these preliminary results, we cannot conclude whether FOXC1 is necessary for the pathogenesis of AM and AOT or whether it is a product of the transcriptional regulation of transformed cells.
Limitation(s)
This work has some limitations, because expression of FOXC1 gene was demonstrated in only one AOT sample. To confirm that FOXC1 is participate in OT, future study may be needed to corroborate these results in other OT. In addition, it is necessary to perform gene expression level and functional studies, such as migration and invasion assays, to analyse the biologic role of FOXC1 protein in OT.
Conclusion(s)
Expression of FOXC1 may contribute in tumourigenesis of OT but not related to pathogenesis of OC. In addition, FOXC1 is not of any function in normal odontogenic development.
Author Declaration:
Financial or Other Competing Interests: As declared above
Was Ethics Committee Approval obtained for this study? Yes
Was informed consent obtained from the subjects involved in the study? NA
For any images presented appropriate consent has been obtained from the subjects. NA
Plagiarism Checking Methods: [Jain H et al.]
Plagiarism X-checker: May 07, 2020
Manual Googling: Jun 17, 2020
iThenticate Software: Aug 27, 2020 (12%)
[1]. Diniz MG, Gomes CC, de Sousa SF, Xavier GM, Gomez RS, Oncogenic signalling pathways in benign odontogenic cysts and tumoursOral Oncol 2017 72:165-73.10.1016/j.oraloncology.2017.07.02128797453 [Google Scholar] [CrossRef] [PubMed]
[2]. Heikinheimo K, Kurppa KJ, Laiho A, Peltonen S, Berdal A, Bouattour A, Early dental epithelial transcription factors distinguish ameloblastoma from keratocystic odontogenic tumourJ Dent Res 2014 94(1):101-11.10.1177/002203451455681525398365 [Google Scholar] [CrossRef] [PubMed]
[3]. Thesleff I, Current understanding of the process of tooth formation: Transfer from the laboratory to the clinicAust Dent J 2014 59(Suppl 1):48-54.10.1111/adj.1210224236691 [Google Scholar] [CrossRef] [PubMed]
[4]. Franca JA, de Sousa SF, Diniz MG, Pereira T, de Resende TAC, Santos JND, Absence of BRAFV600E mutation in odontogenic keratocystsJ Oral Pathol Med 2018 47(2):186-91.10.1111/jop.1267129272070 [Google Scholar] [CrossRef] [PubMed]
[5]. Kurppa KJ, Catón J, Morgan PR, Ristimäki A, Ruhin B, Kellokoski J, High frequency of BRAF V600E mutations in ameloblastomaJ Pathol 2014 232(5):492-98.10.1002/path.431724374844 [Google Scholar] [CrossRef] [PubMed]
[6]. Cha YH, Cho ES, Kang HE, Ko J, Nam W, Kim HJ, Frequent oncogenic BRAF V600E mutation in odontogenic keratocystOral Oncol 2017 74:62-67.10.1016/j.oraloncology.2017.09.01629103753 [Google Scholar] [CrossRef] [PubMed]
[7]. Prasitsak T, Nandar M, Okuhara S, Ichinose S, Ota MS, Iseki S, Foxc1 is required for early stage telencephalic vascular developmentDev Dyn 2015 244(5):703-11.10.1002/dvdy.2426925733312 [Google Scholar] [CrossRef] [PubMed]
[8]. Elian FA, Yan E, Walter MA, FOXC1, the new player in the cancer sandboxOncotarget 2017 9(8)10.18632/oncotarget.2274229487724 [Google Scholar] [CrossRef] [PubMed]
[9]. Inman KE, Purcell P, Kume T, Trainor PA, Interaction between Foxc1 and Fgf8 during mammalian jaw patterning and in the pathogenesis of syngnathiaPLoS Genet 2013 9(12):e100394910.1371/journal.pgen.100394924385915 [Google Scholar] [CrossRef] [PubMed]
[10]. Kume T, Deng KY, Winfrey V, Gould DB, Walter MA, Hogan BL, The forkhead/winged helix gene Mf1 is disrupted in the pleiotropic mouse mutation congenital hydrocephalusCell 1998 93(6):985-96.10.1016/S0092-8674(00)81204-0 [Google Scholar] [CrossRef]
[11]. Liu Z, Xu S, Chu H, Lu Y, Yuan P, Zeng X, Silencing FOXC1 inhibits growth and migration of human oral squamous cell carcinoma cellsExp Ther Med 2018 16(4):3369-76.10.3892/etm.2018.6627 [Google Scholar] [CrossRef]
[12]. Lin Z, Sun L, Chen W, Liu B, Wang Y, Fan S, miR-639 regulates transforming growth factor beta-induced epithelial-mesenchymal transition in human tongue cancer cells by targeting FOXC1Cancer Sci 2014 105(10):1288-98.10.1111/cas.1249925130698 [Google Scholar] [CrossRef] [PubMed]
[13]. Kong XP, Yao J, Luo W, Feng FK, Ma JT, Ren YP, The expression and functional role of a FOXC1 related mRNA-lncRNA pair in oral squamous cell carcinomaMol Cell Biochem 2014 394(1-2):177-86.10.1007/s11010-014-2093-424889262 [Google Scholar] [CrossRef] [PubMed]
[14]. Xu ZY, Ding SM, Zhou L, Xie HY, Chen KJ, Zhang W, FOXC1 contributes to microvascular invasion in primary hepatocellular carcinoma via regulating epithelial-mesenchymal transitionInt J Biol Sci 2012 8(8):1130-41.10.7150/ijbs.476922991501 [Google Scholar] [CrossRef] [PubMed]
[15]. Han B, Bhowmick N, Qu Y, Chung S, Giuliano AE, Cui X, FOXC1: An emerging marker and therapeutic target for cancerOncogene 2017 36(28):3957-63.10.1038/onc.2017.482828814 [Google Scholar] [CrossRef] [PubMed]
[16]. Speight PM, Takata T, New tumour entities in the 4th edition of the World Health Organisation Classification of Head and Neck tumours: odontogenic and maxillofacial bone tumoursVirchows Arch 2018 472(3):331-39.10.1007/s00428-017-2182-328674741 [Google Scholar] [CrossRef] [PubMed]
[17]. Scheper MA, Duarte ECB, Intapa C, Zhang M, Nascimento LM, Almeida TP, Expression of midkine in ameloblastomas and its correlation with clinicopathologic parametersOral Surg Oral Med Oral Pathol Oral Radiol 2012 114(4):497-502.10.1016/j.oooo.2012.06.01322986245 [Google Scholar] [CrossRef] [PubMed]
[18]. Aldinger KA, Lehmann OJ, Hudgins L, Chizhikov VV, Bassuk AG, Ades LC, FOXC1 is required for normal cerebellar development and is a major contributor to chromosome 6p25.3 Dandy-Walker malformationNat Genet 2009 41(9):1037-42.10.1038/ng.42219668217 [Google Scholar] [CrossRef] [PubMed]
[19]. Seo S, Singh HP, Lacal PM, Sasman A, Fatima A, Liu T, Forkhead box transcription factor FoxC1 preserves corneal transparency by regulating vascular growthProc Natl Acad Sci U S A 2012 109(6):2015-20.10.1073/pnas.110954010922171010 [Google Scholar] [CrossRef] [PubMed]
[20]. Liu J, Shen L, Yao J, Li Y, Wang Y, Chen H, Forkhead box C1 promoter upstream transcript, a novel long non-coding RNA, regulates proliferation and migration in basal-like breast cancerMol Med Rep 2015 11(4):3155-59.10.3892/mmr.2014.308925516208 [Google Scholar] [CrossRef] [PubMed]
[21]. Hiemisch H, Monaghan AP, Schutz G, Kaestner KH, Expression of the mouse Fkh1/Mf1 and Mfh1 genes in late gestation embryos is restricted to mesoderm derivativesMech Dev 1998 73(1):129-32.10.1016/S0925-4773(98)00039-2 [Google Scholar] [CrossRef]
[22]. Xiao J, Cao P, Wang C, Huang D, Lian M, Song Y, The Forkhead Box C1, a novel negative regulator of osteogenesis, plays a crucial role in odontogenic differentiation of dental pulp stem cellsCellular Reprogram 2018 20(5):312-19.10.1089/cell.2018.001130277823 [Google Scholar] [CrossRef] [PubMed]
[23]. Levanat S, Pavelić B, Crnić I, Orešković S, Manojlović S, Involvement of PTCH gene in various noninflammatory cystsJ Mol Med 2000 78(3):140-46.10.1007/s00109000009010868476 [Google Scholar] [CrossRef] [PubMed]