Introduction
Periodontitis is initiated by plaque microbes and modified by systemic and environmental factors. Treatment of periodontitis primarily focuses on plaque control by mechanical and chemical means. Chlorhexidine (CHX) mouthwash is considered as the ‘gold standard’ chemical plaque control agent. But studies have demonstrated cytotoxic effects of CHX. However, there is limited evidence available regarding the cytotoxicity of other commonly used postoperative mouthwashes.
Aim
To evaluate cytotoxicity of commonly used postoperative mouthwashes (CHX- 0.12% and 0.2%, 2% povidone iodine, 3% hydrogen peroxide and 0.9% normal saline solutions) using MTT assay on fibroblast cells and to identify the least cytotoxic agent.
Materials and Methods
The study was an invitro study conducted at Department of Periodontics, PMS College of Dental Sciences and Research, Vattapara, Thiruvananthapuram in association with Biogenix research centre Poojapura in January 2018. The cytotoxic effects of CHX -0.12% and 0.2%, Povidone iodine 2%, 3% hydrogen peroxide and 0.9% normal saline solution on L929 fibroblast cells were observed using inverted phase contrast microscope and images were recorded for all the groups. Cytotoxic evaluation was done by MTT {3,(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide} assay. Optical Density (OD) was measured and percentage of cell viability for each mouthwash was calculated. Statistical Analysis was carried out using analysis of variance (ANOVA). Intergroup comparison was done using Post hoc analysis (Tukey HSD). The p-value <0.05 was considered to be statistically significant. SPSS software version 22.0 IBM, Chicago, IL. was used.
Results
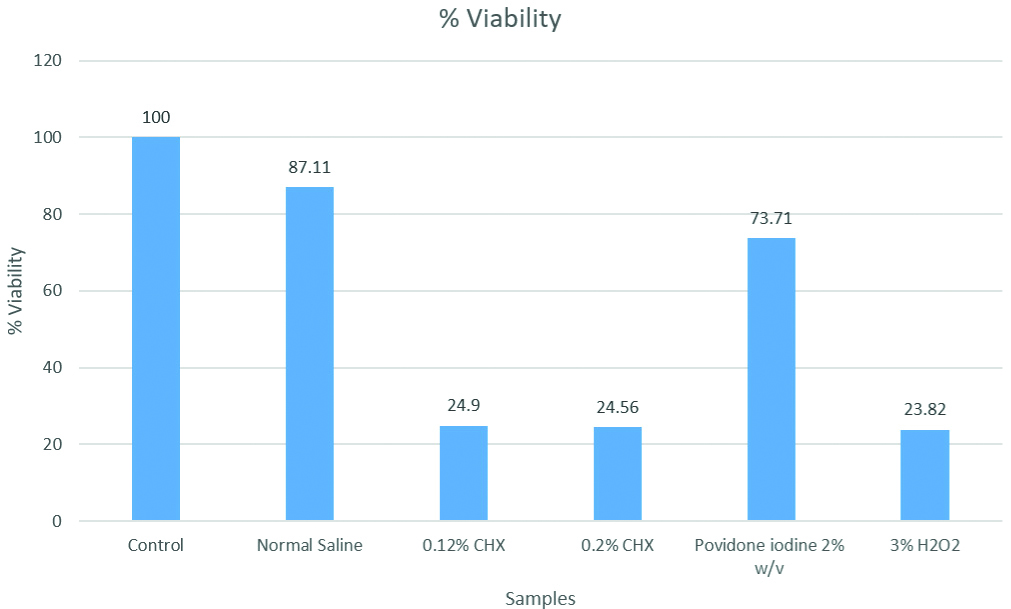
Cell viability percentages were highest for normal saline (87.11%) followed by 2% povidone iodine (73.71%), 0.12% and 0.2% CHX (24.9% and 24.56%) and the least for 3% hydrogen peroxide (23.82%). Post hoc analysis showed significant difference for all the reagents compared to control (p<0.001) except normal saline (p=0.658). The difference between povidone iodine and normal saline was not significant (p=0.433). Comparison of both concentrations of CHX (0.2% and 0.12%) and povidone iodine 2% w/v was significantly different with p<0.001, but not with hydrogen peroxide (3%) (p=0.899). The comparison between povidone iodine 2% and hydrogen peroxide (3%) was significantly different (p<0.001). Microscopic findings of CHX and hydrogen peroxide treated cells included cell shrinkage, condensed nuclei, membrane blebbing and apoptotic bodies. Changes in cellular morphology were not observed in cells treated with povidone iodine and normal saline solution.
Conclusion
Both 0.12% and 0.2% CHX and 3% hydrogen peroxide were found to have significant cytotoxic effects when compared to other mouthwashes. The findings of this study preclude the use of 0.12% and 0.2% CHX and 3% hydrogen peroxide as postoperative mouth rinses due to their possible cytotoxic effects. A 2% povidone iodine and normal saline solution can be considered as excellent alternatives as they were found to be least cytotoxic on fibroblast cells.
Introduction
Periodontitis is a chronic complex inflammatory disease involving the supporting structures of teeth such as periodontal ligament and alveolar bone leading to gingival recession, pocket formation and bone loss [1]. It is a multifactorial disease which is modified by systemic and environmental factors [2]. Oral microbiome in dental plaque plays an unequivocal role in periodontal pathogenesis [3]. Microbial plaque biofilm control is considered to be an established and effective way of treating and preventing periodontal diseases [4]. There are several methods to control the plaque such as the mechanical and chemical plaque control [5]. Ideally, any antimicrobial agent that is used, should be able to alter the oral environment in such a way that it is effective against the pathogens without altering the tissue homeostasis. [6]. Chemical plaque control agents can be delivered in the form of mouthwashes, ointments, local drug delivery system etc., [5].
Among the chemicals used in mouthwashes, CHX is the most commonly prescribed and most potent plaque control agent [7]. CHX is a cationic bisbiguanide which is effective against gram positive, gram negative organisms, fungi, yeasts and viruses; and exhibit antiplaque and antibacterial properties [8]. Commonly used concentrations of commercially available CHX mouthwashes are 0.12% and 0.2%. But there are some reported disadvantages to the use of CHX like staining of teeth and oral tissues, taste perturbation where the salt taste appears to be preferentially affected to leave food and drinks with a bland taste, oral mucosal erosion, bilateral parotid swelling, and enhanced supragingival calculus formation [9]. In the past, CHX has been identified to be cytotoxic for human gingival fibroblasts, osteocytes and blood cells [10]. CHX is often prescribed after periodontal surgical procedures for postoperative plaque control and the cytotoxicity of CHX has created a confusion in this regard [11,12].
Povidone iodine is another commonly prescribed mouth rinse with antifungal, antiviral and bactericidal properties. It is considered to be less toxic, does not stain and cause any allergic reactions or tissue irritation [13]. Povidone iodine also has been used in wound care because of its broad antibacterial spectrum, efficacy against bacterial biofilms, effects on inflammation, lack of bacterial resistance and promotion of healing by activation of monocytes, T cells, and macrophages [14].
Hydrogen Peroxide (H2O2) levels above 1% have been found to have beneficial effects. A 1.5% H2O2 is used as an adjunct to CHX in reducing plaque and in preventing stain development [15]. The therapeutic action of hydrogen peroxide is obtained by the release of oxygen that kills the putative anaerobes in oral infections [15]. A 3% H2O2 is reported to have a beneficial effect in reducing gingival inflammation and pocket depth [16]. Normal saline solution is effective in preserving gingival health, promoting healing of oral ulcers and is also used as a postoperative mouth rinse [17]. But there is limited evidence available on the cytotoxicity of povidone iodine, hydrogen peroxide and normal saline solution. So, the aim of the present study was to evaluate and compare cytotoxic effect of 0.12% and 0.2% CHX with 3% hydrogen peroxide, povidone iodine 2% solution and 0.9% normal saline solution.
Materials and Methods
The study was an invitro study conducted at Department of Periodontics, PMS College of Dental Sciences and Research, Vattapara, Thiruvananthapuram in association with Biogenix research centre Poojapura in January 2018. Since the study was purely an invitro study we haven’t obtained IEC.
For this study, commonly used postoperative mouthwashes i.e., 0.12% and 0.2% CHX, 2% povidone iodine, 3% hydrogen peroxide, 0.9% normal saline solution and growth medium alone Dulbecos Modified Eagles Medium (DMEM)) as the control have been used. Normal saline solution was prepared by diluting 9 g of salt in 1 litre of water. L929 (adipose tissue Fibroblast) cells were procured from National Centre for Cell Sciences (NCCS), Pune, India and maintained in DMEM (From Gibco, Invitrogen). Cytotoxicity was measured by MTT assay (colorimetric assay) [18]. It’s based on the property of the living cells to reduce soluble yellow tetrazolium salts to blue formazan crystals [18]. International organisation for standardisation has recommended the use of L929, CCL163, CCL171 and SaOS2 cell lines for the evaluation of cytotoxicity of dental materials in invitro studies [19]. Among these L929 cell line has been widely used [19].
The methodology followed was as described previously [20]. Culturing of the cell line was done using tissue culture flask of 25 cm2 containing DMEM which is supplemented with 10% Fetal Bovine Serum (FBS), L-glutamine, sodium bicarbonate and antibiotic solution. Antibiotics used were Penicillin (100 U/mL), Streptomycin (100 μg/mL), and Amphotericin B (2.5 μg/mL). Cell lines were cultured in a 5% CO2 incubator (NBS Eppendorf, Germany) at 37°C. Two-day-old confluent monolayer of cells were allowed to trypsinise and the cells were suspended in a 10% growth medium, 100 μL cell suspension with a density of (5×104 cells/well) was seeded in a 96 well tissue culture plate and further incubated at 37°C in a humidified 5% CO2 incubator and allowed to reach the exponential phase of growth (cell counting was done using Haemocytometer, Sigma).
Cytotoxicity Evaluation
Cells were treated with freshly prepared 0.9% normal saline solution, 2% povidone iodine, 3% hydrogen peroxide, 0.12% CHX and 0.2% CHX and kept for 24 hours in an incubator at 37°C. Wells maintained with growth medium alone was considered as the control. Experiment with each chemical was repeated in 5 wells.
Cytotoxicity Assay by Direct Microscopic Observation
Inverted phase contrast microscope (Olympus CKX41 with Optika Pro5 CCD camera) was used for direct microscopic examination after treatment with chemicals for 24 hours and the observation were recorded as images. Any alteration in cell morphology or shape, such as rounding, cell shrinkage, condensed nuclei, granulation and vacuolisation in the cytoplasm of the cells, appearance of membrane blebbing and apoptotic bodies were considered as indicators of cytotoxicity.
Cytotoxicity Assay by MTT Method
MTT {3,(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide} was then added at a final concentration of 500 μg/mL. The amount of formazan crystals formed were measured after 4 hours of MTT addition [Table/Fig-1]. The crystals were dissolved in isopropyl alcohol and OD was measured at 540 nm. The percentage of viable cells/well was estimated by the following calculation: Viability of cells (%)=(Absorbance of treated cells)/(Absorbance of untreated cells)×100 [20].
Statistical Analysis
Data were represented as mean±standard deviation and percentage. SPSS software version 22.0 IBM, Chicago, IL was used. Statistical Analysis was carried out using analysis of variance (ANOVA) for intergroup comparison along with Post hoc analysis (Tukey HSD). A p-value <0.05 was considered to be statistically significant in all the comparisons. Analysis was done with software.
Results
Mean optical densities and results of statistical analysis (ANOVA) of intergroup comparison between control, normal saline solution, 0.12% CHX, 0.2% CHX, 2% povidone iodine and 3% hydrogen peroxide solutions are represented in [Table/Fig-2]. The difference was found to be statistically significant with p<0.001.
[Table/Fig-3] shows results of Post hoc analysis to find out where the difference occurred between specific groups. Post hoc analysis showed significant difference for all the reagents compared to control (p<0.001) except normal saline (p=0.658). The difference between povidone iodine and normal saline was not significant (p=0.433). Comparison of both concentrations of CHX (0.2% and 0.12%) and Povidone iodine 2% w/v was significantly different with p<0.001, but not with hydrogen peroxide (3%) (p=0.899). The comparison between Povidone iodine 2% and hydrogen peroxide (3%) was significantly different (p<0.001).
Intergroup comparison of mean Optical Density (OD) of different mouthwashes using ANOVA.
| Parameters | Mean OD (nm) | SD | f-value | p-value |
|---|
| Control | 0.8048 | 0.1164 | 71.8618 | p<0.001** |
| Normal saline | 0.7011 | 0.0274 |
| 0.12% CHX | 0.2004 | 0.0147 |
| 0.2% CHX | 0.1977 | 0.0461 |
| povidone iodine 2% | 0.5932 | 0.0446 |
| 3% H2O2 | 0.1917 | 0.0389 |
*Statistically significant at p<0.05 **Statistically highly significant at p<0.001
Post hoc analysis (Tukey HSD) for comparison of specific groups.
| Treatment groups | Tukey HSD p-value |
|---|
| Control vs normal saline | 0.658 |
| Control vs 0.2% CHX | <0.001** |
| Control vs 0.12% CHX | <0.001** |
| Control vs Povidone iodine 2% w/v | <0.001** |
| Control vs 3% hydrogen peroxide | <0.001** |
| Normal saline vs 0.12% CHX | <0.001** |
| Normal saline vs 0.2%CHX | <0.001** |
| Normal saline vs Povidone iodine 2% w/v | 0.433 |
| Normal saline vs 3% hydrogen peroxide | <0.001** |
| 0.2% CHX vs 0.12% CHX | 0.899 |
| 0.2% CHX vs Povidone iodine 2% w/v | <0.001** |
| 0.2% CHX vs 3% hydrogen peroxide | 0.899 |
| 0.12% CHX vs Povidone iodine 2% w/v | <0.001** |
| 0.12% CHX vs 3% hydrogen peroxide | 0.899 |
| 2% povidone iodine w/v vs 3% hydrogen peroxide | <0.001** |
*Statistically significant at p<0.05 **Statistically highly significant at p<0.001
From [Table/Fig-4] cell viability percentages were found to be highest in 0.9% normal saline solution (87.11%) followed by 2% povidone iodine w/v (73.71%) whereas, it was lowest for CHX formulations of both concentrations (24.9% and 24.56%) followed by 3% hydrogen peroxide (23.82%).
Graphical representation of percentage of cell viability.
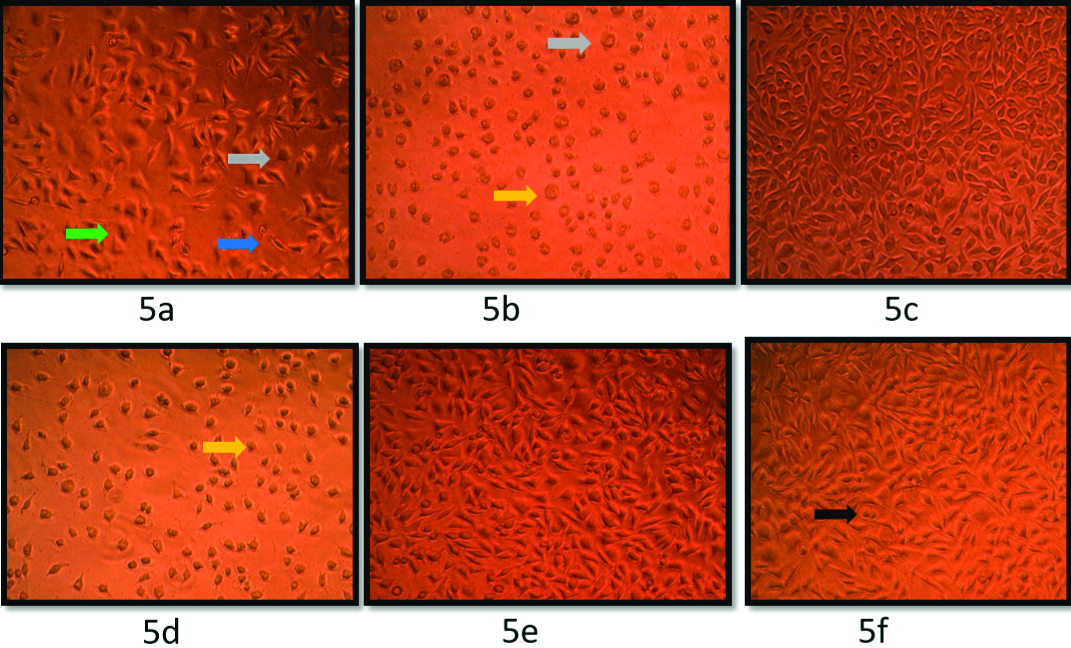
[Table/Fig-5] demonstrates microscopic examination revealing cytotoxic changes like necrosis and rounding of cells in wells treated with hydrogen peroxide and CHX whereas normal cellular morphology was maintained in all other groups.
Microscopic examination of fibroblasts after 24 hours of treatment with different mouthwashes: (5a) 0.2% chlorhexidine; (5b) 0.12% chlorhexidine; (5c) 2% povidone iodine w/v; (5d) 3% hydrogen peroxide; (5e) 0.9% normal saline solution; (5f) Control (Blue arrow- cell shrinkage, yellow- condensed nuclei, Green- Membrane blebbing, grey- apoptotic bodies, Black arrow- Normal unaltered cell from the growth medium).
Discussion
The present study was conducted to evaluate and compare the cytotoxic effects of commonly used postoperative mouthwashes (0.2% and 0.12% CHX, 2% povidone iodine, 3% hydrogen peroxide and normal saline solution) using microscopic examination and MTT assay and a statistically highly significant difference p<0.001 was obtained on comparing intergroup optical densities. Comparison between the groups shows significantly higher cytotoxicity for all the reagents compared to control (p<0.001). When compared to normal saline, cytotoxicity of povidone iodine was comparable (p=0.433). Cytotoxicity of both concentration of CHX (0.2% and 0.12%) and hydrogen peroxide (3%) was significantly higher compared to povidone iodine (p=0.899). In the present study, the cell viability percentages found in descending order were for saline, 2% povidone iodine, 0.2% and 0.12% CHX and 3% hydrogen peroxide. Microscopic findings of CHX and hydrogen peroxide treated cells included cell shrinkage, condensed nuclei, membrane blebbing and apoptotic bodies. Changes in cellular morphology were not observed in cells treated with povidone iodine and normal saline solution.
The cytotoxic effect of CHX on fibroblasts was reported previously in many other studies. Alleyn CD et al., found that a 3 minute exposure of 0.12% CHX had a cytotoxic effect on periodontal ligament fibroblasts [21]. Wilken R et al., demonstrated that there was an immediate cell fixation into tissue cell surfaces when exposed to 0.2% CHX [22]. Louis SM and Pearson RM, in his study demonstrated cell membrane disruption and fixation of cytoplasmic contents of PMNs when exposed to 0.2% CHX [23]. Here, similar results were obtained with 0.2% and 0.12% CHX.
Studies by Chang YC et al., and Rajabalian S et al., found that CHX was cytotoxic to cultured human periodontal ligament and gingival fibroblasts even at a very low concentration (0.0001% or greater) [24,25]. In another study by Coelho AS et al., cytotoxicity of CHX was found to be higher than that of an enzymatic mouthwash containing lysozyme, lactoferrin and lactoperoxidase [26]. Coelho AS et al., observed block in G2/M phase and cell death by necrosis due to CHX [26]. It has also been observed in other studies that CHX impairs human fibroblast attachment to the root surfaces [27] and thus affects cellular proliferation and total protein production when examined invitro [28]. The present study results are in agreement with several authors who associated CHX with cytotoxic effects in gingival epithelial cells [29], in periodontal ligament cells [24], in keratinocytes [30,31], in macrophages [32], in osteoblasts and in osteoclasts [33-35]. Several studies have also reported that CHX causes lysis of erythrocytes and neutrophils [36,37], protein synthesis is inhibited in fibroblasts [28,38] and fibroblasts adhesion and production of matrix components were also reduced [10,39]. Eren K et al., in their study had used 0.12% CHX mouthwash twice a day for 18 days and reported DNA changes in oral epithelial cells and lymphocytes [40].
Flemingson et al., evaluated the cytotoxicity of CHX mouthwash according to concentration of mouthwash and time of exposure [12]. They evaluated the metabolic activity of human gingival fibroblasts after exposing the cells to CHX mouthwash at different concentrations for 1 minute, 5 minutes and 15 minutes. A 51.7% reduction in the metabolic activity after 1 minute of exposure to CHX (0.002%), 62.1% reduction after 5 minutes and 72.6% reduction after 15 minutes were reported. Using concentrations equal to or higher than 0.02%, the metabolic activity was reduced by more than 90% in all of the tested groups.
In the present study, 2% povidone iodine had significantly less cytotoxicity (73.71% cell viability) compared to H2O2 and CHX. But there are conflicting results that povidone iodine can be cytotoxic at concentration of 5.0% to 0.05% when tested in canine embryonic fibroblasts [41] and at concentration of 1% in human gingival fibroblasts [42]. In a study, by Lineaweaver W et al., suggested that a concentration of 0.001% has bactericidal activity and it has no toxic effect on human fibroblasts [43].
In the present study, hydrogen peroxide was found to have greatest cytotoxicity than other agents. This was in accordance with other studies conducted by Palmqvist P et al., and Kiyoshima T et al., in which they demonstrated the cytotoxicity of H2O2 to Gingival fibroblasts at a concentration of 50 μM [44,45]. In another study by Furukawa M et al., highly concentrated (15%) H2O2 caused inflammation in gingival fibroblasts and had toxic effects which also represent marked changes in cell morphology [46].
In an invitro study by Huynh NC et al., the findings were in accordance with the present study. It was concluded that the use of saline enhances the wound healing capacity and has a beneficial effect on human gingival fibroblasts [17]. Saline solution enhances human gingival fibroblast cell migration, alters the structure of cytoskeletal molecules and enhances extracellular matrix gene expression.
Limitation(s)
The present study was an invitro study and controlled clinical trials should be conducted in future to verify the present study findings. The clinical efficacy of the tested agents should also be compared in terms of antiplaque and anti-inflammatory effects to select the most appropriate mouthwash for postoperative use. Another limitation was the lack of use of human gingival fibroblasts instead of L929. Due to the lack of the facility of human gingival fibroblasts, future studies can be conducted with the same to overcome these drawbacks.
Conclusion(s)
On comparing the cytotoxicity of commonly used postoperative mouthwashes, it was demonstrated that cytotoxic effects of normal saline and 2% povidone iodine were significantly less compared to 0.12% and 0.2% CHX as well as 3% hydrogen peroxide. Even though CHX is considered as the gold standard antiplaque agent, we have to be cautious when we prescribe it during immediate postoperative period. Based on the results of the present study, 2% povidone iodine and normal saline solution as excellent alternative to CHX for postoperative use could be recommended. Future clinical studies can provide more evidence in this regard.
*Statistically significant at p<0.05 **Statistically highly significant at p<0.001
*Statistically significant at p<0.05 **Statistically highly significant at p<0.001
[1]. Armitage GC, Development of a classification system for periodontal diseases and conditionsAnn Periodontol 1999 4(1):01-06.10.1902/annals.1999.4.1.110863370 [Google Scholar] [CrossRef] [PubMed]
[2]. Taba Jr M, Souza SL, Mariguela VC, Periodontal disease: A genetic perspectiveBraz Oral Res 2012 26(SPE1):32-38.10.1590/S1806-8324201200070000623318742 [Google Scholar] [CrossRef] [PubMed]
[3]. Bartold PM, Van Dyke TE, Periodontitis: A host mediated disruption of microbial homeostasis. Unlearning learned conceptsPeriodontol 2000 2013 62(1):203-17.10.1111/j.1600-0757.2012.00450.x23574467 [Google Scholar] [CrossRef] [PubMed]
[4]. Löe H, Plaque control in periodontal diseaseJ Am Dent Assoc 1973 87(5):1034-36.10.14219/jada.archive.1973.00114518337 [Google Scholar] [CrossRef] [PubMed]
[5]. Figuero E, Nobrega DF, García Gargallo M, Tenuta LM, Herrera D, Carvalho JC, Mechanical and chemical plaque control in the simultaneous management of gingivitis and caries: A systematic reviewJ Clin Periodontol 2017 44:S116-34.10.1111/jcpe.1267428266113 [Google Scholar] [CrossRef] [PubMed]
[6]. Albert Kiszely A, Pjetursson BE, Salvi GE, Witt J, Hamilton A, Persson GR, Comparison of the effects of cetylpyridinium chloride with an essential oil mouth rinse on dental plaque and gingivitis- A six-month randomized controlled clinical trialJ Clin Periodontol 2007 34(8):658-67.10.1111/j.1600-051X.2007.01103.x17635245 [Google Scholar] [CrossRef] [PubMed]
[7]. Balagopal S, Arjunkumar R, Chlorhexidine: The gold standard antiplaque agentJournal of Pharmaceutical sciences and Research 2013 5(12):270 [Google Scholar]
[8]. Parwani SR, Parwani RN, Chitnis PJ, Dadlani HP, Prasad SV, Comparative evaluation of anti-plaque efficacy of herbal and 0.2% chlorhexidine gluconate mouthwash in a 4-day plaque re-growth studyJournal of Indian Society of Periodontology 2013 17(1):7210.4103/0972-124X.10747823633777 [Google Scholar] [CrossRef] [PubMed]
[9]. Flötra L, Gjermo PE, Rölla G, Waerhaug J, Side effects of chlorhexidine mouth washesEur J Oral Sci 1971 79(2):119-25.10.1111/j.1600-0722.1971.tb02001.x5280246 [Google Scholar] [CrossRef] [PubMed]
[10]. Mariotti AJ, Rumpf DA, Chlorhexidine induced changes to human gingival fibroblast collagen and non-collagen protein productionJ Periodontol 1999 70(12):1443-48.10.1902/jop.1999.70.12.144310632519 [Google Scholar] [CrossRef] [PubMed]
[11]. Dona BL, Gründemann LJ, Steinfort J, Timmerman MF, Van der Weijden GA, The inhibitory effect of combining chlorhexidine and hydrogen peroxide on 3-day plaque accumulationJ Clin Periodontol 1998 25(11):879-83.10.1111/j.1600-051X.1998.tb02385.x9846796 [Google Scholar] [CrossRef] [PubMed]
[12]. Flemingson Emmadi P, Ambalavanan N, Ramakrishnan T, Vijayalakshmi R, Effect of three commercial mouth rinses on cultured human gingival fibroblast: An in vitro studyIndian J Dent Res 2008 19(1):2910.4103/0970-9290.3892918245921 [Google Scholar] [CrossRef] [PubMed]
[13]. Hasheminia D, Moaddabi A, Moradi S, Soltani P, Moannaei M, Issazadeh M, The efficacy of 1% betadine mouthwash on the incidence of dry socket after mandibular third molar surgeryJ Clin Exp Dent 2018 10(5):e44510.4317/jced.5444429849968 [Google Scholar] [CrossRef] [PubMed]
[14]. Bigliardi PL, Alsagoff SA, El-Kafrawi HY, Pyon JK, Wa CT, Villa MA, Povidone iodine in wound healing: A review of current concepts and practicesInternational Journal of Surgery 2017 44:260-68.10.1016/j.ijsu.2017.06.07328648795 [Google Scholar] [CrossRef] [PubMed]
[15]. Jhingta P, Bhardwaj A, Sharma D, Kumar N, Bhardwaj VK, Vaid S, Effect of hydrogen peroxide mouthwash as an adjunct to chlorhexidine on stains and plaqueJ Indian Soc Periodontol 2013 17(4):44910.4103/0972-124X.11831524174723 [Google Scholar] [CrossRef] [PubMed]
[16]. Wolff LF, Bandt C, Pihlstrom B, Brayer L, Phase contrast microscopic evaluation of subgingival plaque in combination with either conventional or antimicrobial home treatment of patients with periodontal inflammationJournal of Periodontal Research 1982 17(5):537-40.10.1111/j.1600-0765.1982.tb02050.x6296347 [Google Scholar] [CrossRef] [PubMed]
[17]. Huynh NC, Everts V, Leethanakul C, Pavasant P, Ampornaramveth RS, Rinsing with saline promotes human gingival fibroblast wound healing in vitroPloS one 2016 11(7)10.1371/journal.pone.015984327441729 [Google Scholar] [CrossRef] [PubMed]
[18]. Li YC, Kuan YH, Lee TH, Huang FM, Chang YC, Assessment of the cytotoxicity of chlorhexidine by employing an in vitro mammalian test systemJ Dent Sci 2014 9(2):130-35.10.1016/j.jds.2013.02.011 [Google Scholar] [CrossRef]
[19]. ISO B, 10993-5: Biological evaluation of medical devicesTests for in vitro cytotoxicity 1999 [Google Scholar]
[20]. Ambili R, Janam P, Babu PS, Prasad M, Vinod D, Kumar PA, An ex vivo evaluation of the efficacy of andrographolide in modulating differential expression of transcription factors and target genes in periodontal cells and its potential role in treating periodontal diseasesJ Ethnopharmacol 2017 196:160-67.10.1016/j.jep.2016.12.02927993634 [Google Scholar] [CrossRef] [PubMed]
[21]. Alleyn CD, O’Neal RB, Strong SL, Scheidt MJ, Van Dyke TE, McPherson JC, The effect of chlorhexidine treatment of root surfaces on the attachment of human gingival fibroblasts in vitroJ Periodontol 1991 62(7):434-38.10.1902/jop.1991.62.7.4341920010 [Google Scholar] [CrossRef] [PubMed]
[22]. Wilken R, Botha SJ, Grobler A, Germishuys PJ, In vitro cytotoxicity of chlorhexidine gluconate, benzydamine-HCl and povidone iodine mouthrinses on human gingival fibroblastsSADJ 2001 56(10):455-60. [Google Scholar]
[23]. Louis SM, Pearson RM, A comparison of the effects of nonoxynol-9 and chlorhexidine on sperm motilityContraception 1985 32(2):199-205.10.1016/0010-7824(85)90108-8 [Google Scholar] [CrossRef]
[24]. Chang YC, Huang FM, Tai KW, Chou MY, The effect of sodium hypochlorite and chlorhexidine on cultured human periodontal ligament cellsOral Surg Oral Med Oral Pathol Oral Radiol Endod 2001 92(4):446-50.10.1067/moe.2001.11681211598582 [Google Scholar] [CrossRef] [PubMed]
[25]. Rajabalian S, Mohammadi M, Mozaffari B, Cytotoxicity evaluation of Persica mouthwash on cultured human and mouse cell lines in the presence and absence of fetal calf serumIndian J Dent Res 2009 20(2):16910.4103/0970-9290.5289419553717 [Google Scholar] [CrossRef] [PubMed]
[26]. Coelho AS, Laranjo M, Goncalves AC, Paula A, Paulo S, Abrantes AM, Cytotoxic effects of a chlorhexidine mouthwash and of an enzymatic mouthwash on human gingival fibroblastsOdontology 2020 108(2):260-70.10.1007/s10266-019-00465-z31624978 [Google Scholar] [CrossRef] [PubMed]
[27]. Cline NV, Layman DL, The effects of chlorhexidine on the attachment and growth of cultured human periodontal cellsJ Periodontol 1992 63(7):598-602.10.1902/jop.1992.63.7.5981507037 [Google Scholar] [CrossRef] [PubMed]
[28]. Pucher JJ, Daniel C, The effects of chlorhexidine digluconate on human fibroblasts in vitroJ Periodontol 1992 63(6):526-32.10.1902/jop.1992.63.6.5261625152 [Google Scholar] [CrossRef] [PubMed]
[29]. Babich H, Wurzburger BJ, Rubin YL, Sinensky MC, Blau L, An in vitro study on the cytotoxicity of chlorhexidine digluconate to human gingival cellsCell Biol Toxicol 1995 11(2):79-88.10.1007/BF007674937583874 [Google Scholar] [CrossRef] [PubMed]
[30]. Damour O, Hua SZ, Lasne F, Villain M, Rousselle P, Collombel C, Cytotoxicity evaluation of antiseptics and antibiotics on cultured human fibroblasts and keratinocytesBurns 1992 18(6):479-85.10.1016/0305-4179(92)90180-3 [Google Scholar] [CrossRef]
[31]. Tatnall FM, Leigh IM, Gibson JR, Comparative study of antiseptic toxicity on basal keratinocytes, transformed human keratinocytes and fibroblastsSkin Pharmacol Physiol 1990 3(3):157-63.10.1159/0002108652078350 [Google Scholar] [CrossRef] [PubMed]
[32]. Segura JJ, Jiménez-Rubio A, Guerrero JM, Calvo JR, Comparative effects of two endodontic irrigants, chlorhexidine digluconate and sodium hypochlorite, on macrophage adhesion to plastic surfacesJ Endod 1999 25(4):243-46.10.1016/S0099-2399(99)80151-4 [Google Scholar] [CrossRef]
[33]. Giannelli M, Chellini F, Margheri M, Tonelli P, Tani A, Effect of chlorhexidine digluconate on different cell types: a molecular and ultrastructural investigationToxicol In Vitro 2008 22(2):308-17.10.1016/j.tiv.2007.09.012 [Google Scholar] [CrossRef]
[34]. Cabral CT, Fernandes MH, In vitro comparison of chlorhexidine and povidone-iodine on the long-term proliferation and functional activity of human alveolar bone cellsClin Oral Investig 2007 11(2):155-64.10.1007/s00784-006-0094-817216529 [Google Scholar] [CrossRef] [PubMed]
[35]. Bhandari M, Adili A, Schemitsch EH, The efficacy of low-pressure lavage with different irrigating solutions to remove adherent bacteria from boneJ Bone Joint Surg Am 2001 83(3):41210.2106/00004623-200103000-0001411263646 [Google Scholar] [CrossRef] [PubMed]
[36]. Helgeland K, Heyden G, Rölla G, Effect of chlorhexidine on animal cells in vitroEur J Oral Sci 1971 79(2):209-15.10.1111/j.1600-0722.1971.tb02011.x4105986 [Google Scholar] [CrossRef] [PubMed]
[37]. Gabler WL, Roberts D, Harold W, The effect of chlorhexidine on blood cellsJournal of periodontal research 1987 22(2):150-55.10.1111/j.1600-0765.1987.tb01555.x3035162 [Google Scholar] [CrossRef] [PubMed]
[38]. Goldschmidt P, Cogen R, Taubman S, Cytopathologic effects of chlorhexidine on human cellsJ Periodontol 1977 48(4):212-15.10.1902/jop.1977.48.4.212265388 [Google Scholar] [CrossRef] [PubMed]
[39]. Balloni S, Locci P, Lumare A, Marinucci L, Cytotoxicity of three commercial mouthrinses on extracellular matrix metabolism and human gingival cell behaviourToxicol In Vitro 2016 34:88-96.10.1016/j.tiv.2016.03.01527039991 [Google Scholar] [CrossRef] [PubMed]
[40]. Eren K, Ízmeriš N, Şardaş S, Monitoring of buccal epithelial cells by alkaline comet assay (single cell gel electrophoresis technique) in cytogenetic evaluation of chlorhexidineClin Oral Investig 2002 6(3):150-54.10.1007/s00784-002-0168-112271347 [Google Scholar] [CrossRef] [PubMed]
[41]. Sanchez IR, Nusbaum KE, Swaim SF, Hale AS, Henderson RA, Mcguire JA, Chlorhexidine diacetate and povidone iodine cytotoxicity to canine embryonic fibroblasts and Staphylococcus aureusVet Surg 1988 17(4):182-85.10.1111/j.1532-950X.1988.tb00995.x3238890 [Google Scholar] [CrossRef] [PubMed]
[42]. Barnhart BD, Chuang A, Dalle Lucca JJ, Roberts S, Liewehr F, Joyce AP, An in vitro evaluation of the cytotoxicity of various endodontic irrigants on human gingival fibroblastsJ Endod 2005 31(8):613-15.10.1097/01.don.0000153840.94227.8716044047 [Google Scholar] [CrossRef] [PubMed]
[43]. Lineaweaver W, McMorris S, Soucy D, Howard R, Cellular and bacterial toxicities of topical antimicrobialsPlast Reconstr Surg 1985 75(3):394-96.10.1097/00006534-198503000-000163975287 [Google Scholar] [CrossRef] [PubMed]
[44]. Palmqvist P, Lundberg P, Lundgren I, Hänström L, Lerner UH, IL-1β and TNF-α regulate IL-6-type cytokines in gingival fibroblastsJ Dent Res 2008 87(6):558-63.10.1177/15440591080870061418502965 [Google Scholar] [CrossRef] [PubMed]
[45]. Kiyoshima T, Enoki N, Kobayashi I, Sakai T, Nagata K, Wada H, Oxidative stress caused by a low concentration of hydrogen peroxide induces senescence-like changes in mouse gingival fibroblastsInt J Mol Med 2012 30(5):1007-12.10.3892/ijmm.2012.110222922974 [Google Scholar] [CrossRef] [PubMed]
[46]. Furukawa M, K-Kaneyama J, Yamada M, Senda A, Manabe A, Miyazaki A, Cytotoxic effects of hydrogen peroxide on human gingival fibroblasts in vitroOper Dent 2015 40(4):430-39.10.2341/14-059-L25575199 [Google Scholar] [CrossRef] [PubMed]