Detection of Rifampicin Resistance among Patients with Tuberculosis using GeneXpert MTB/RIF Assay: A Retrospective Study
Gayathridevi Durairaj1, Sridhar Rathinam2, Vinodkumar Vishwanathan3, Kumar Satagopan4
1 Tutor, Department of Microbiology, Stanley Medical College, Chennai, Tamil Nadu, India.
2 Professor and Head, Department of Pulmonology, Stanley Medical College, Chennai, Tamil Nadu, India.
3 Professor, Department of Pulmonology, Stanley Medical College, Chennai, Tamil Nadu, India.
4 Nodal Officer, Department of TB Center, Government Hospital of Thoracic Medicine, Chennai, Tamil Nadu, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Gayathridevi Durairaj, Tutor, Department of Microbiology, Stanley Medical College, Chennai, Tamil Nadu, India.
E-mail: gayathridevidurairaj@gmail.com
Introduction
Tuberculosis (TB) remains one of the major cause of death worldwide, and the leading causes of mortality in developing countries like India. Smear microscopy has certain limits as it requires 10,000 bacilli/mL for positivity, culture methods takes longer turnaround time of around 6-8 weeks. The recent advancement like Genexpert Mycobacterium tuberculosis/Rifampicin (MTB/RIF) Assay, which has more accuracy and analysis where it detects MTB and RIF resistance in smear negative including immunosuppressive patients even with a volume of 138 cfu (colony forming units)/mL in less than two hours. World Health Organisation (WHO) also recommended new Cartridge Based Nucleic Acid Amplification Test (CB-NAAT) on 2010 and named as GeneXpert system for diagnosis of TB.
Aim
To evaluate the patterns of RIF Resistance by GeneXpert as a Rapid and primary screening test in TB patients.
Materials and Methods
Both pulmonary and extra pulmonary samples were subjected to AFB (Acid Fast Bacilli) smear microscopy before being tested for GeneXpert MTB/RIF Assay. MTB detected samples were further tested at National Institute for Research in Tuberculosis (NIRT) to confirm RIF resistance and to find out associated resistance to Isoniazid and other second line drugs like fluroquinolones, kanamycin, capreomycin and amikacin. All statistical analysis were performed using Statistical Package for the Social Sciences (SPSS) version 15.0.
Results
A total of 8140 samples were tested for CB-NAAT, MTB was not detected in 4414 samples, MTB detected and RIF Sensitive in 3554 samples, MTB detected and RIF resistance in 172 samples. Among these RIF Resistant samples 97% were retreatment patients and the primary drug resistance was less common. HIV/TB co-infected contributes to 1% of resistance and there was no gender preponderance. RIF mono-resistance was found in majority of patients.
Conclusion
Genexpert has higher specificity for early detection of RIF resistance as a surrogate marker of MDR-TB to initiate early treatment and prevent transmission of Multi Drug Resistance (MDR) strains.
Cartridge based nucleic acid amplification test, Multidrug resistance tuberculosis, Mycobacterium tuberculosis
Introduction
Worldwide, TB is one of the top 10 causes of death and the leading cause from a single infectious agent (above HIV/AIDS). About 10 million people are infected with TB worldwide, only seven million reported have access with TB care and three milion people remain undiagnosed, report from WHO 2019 [1]. Global TB Report from WHO shows, a total of 1.5 million people died from TB in 2018 (including 251000 people with HIV). In 2018, India was one of the 30 high TB burden countries which accounted for 87% of new TB cases. Eight countries account for two thirds of the total, with India leading the count followed by, China, Indonesia, the Philippines, Pakistan, Nigeria, Bangladesh and South Africa. Drug Resistant TB is caused by dangerous mutant TB bacteria which require diagnostic laboratory tests that are inaccessible for the patients [1]. Only one out of five patients are diagnosed as MDR. India ranks second to China in having highest number of MDR cases and 99,000 new cases occur annually [1,2]. Revised National Tuberculosis Control Program (RNTCP) has executed many plans to improve the number and quality of diagnostic i.e., culture and Drug Susceptibility Testing (DST) laboratories and as well as Directly Observed Treatment, Short-course (DOTS) for the detection and treatment of MDR among new and previously treated TB patients [3]. But burden of MDR TB is still increasing due to slow diagnostic aids, inaccessibility to health care, inadequate treatment and shortage of trained persons.
RIF is a semi-synthetic antibiotic produced from Streptomyces mediterrani. RIF resistance is one of the surrogate markers for MDR TB as it is often associated with isoniazid resistance. RIF resistance is due to mutations of the rpoB gene at the 81 bp region that codes for b-subunit of RNA polymerase [4]. Most of the mutations occur at the codons 516, 526 and 531 of the Rifampicin Resistance Determining Region (RRDR), also called as hot spot region [4,5]. Mutations within this hot spot region confer high level of resistance to RIF. In rpoB gene mutation where the base in the DNA is replaced by new one and the new sequence codes for an amino acid with larger side chain that inhibits RIF molecules from binding to RNA polymerase and thus synthesis of proteins needed for survival of bacilli, causing drug resistance.
GeneXpert is a sensitive and specific test [6] for rapid and early diagnosis of TB as well as resistance to RIF in less than two hours. The GeneXpert MTB/RIF Assay is an automated nested Real Time Polymerase Chain Reaction (RT-PCR) test which uses three primers and molecular beacons in five overlapping regions of the rpoB DNA region [2]. The probes are able to detect mutations in the codons 507 to 511 (Probe A), 511 to 518 (Probe B), 518 to 523 (Probe C), 523 to 529 (Probe D), and 529 to 533 (Probe E) [7] GeneXpert is preferred over conventional culture and DST methods as it takes longer turnaround time of 8 to 12 weeks. GeneXpert MTB/RIF Assay detects MTB by identifying its DNA and RIF Resistance by mutant rpoB gene. It purifies and concentrates MTB bacilli from samples, isolates genomic material by sonication and subsequently amplified by PCR. This process also identifies mutations in the rpoB gene by using fluorescent probes called molecular beacons in a real time format. Results are available in 90 minutes. GeneXpert can be preferred over conventional culture and DST methods as it needs appropriate lab infrastructure and more complex procedures, expensive and longer turn time of 6-8 weeks [2,6]. There is an increase in global notification of new TB patients since 2013 is because of increased notification in India and Indonesia, but lesser than the actual number of estimated cases. In India notification of new cases, increased from 1.2 to 2.0 million between 2013 and 2018 [1]. Even though there is increase in TB notification, still there is gap between number of new case reported and estimated case. This might be due to combination of underreporting of detected cases and under diagnosis. So the current study was aimed to evaluate the patterns of RIF resistance by GeneXpert as a rapid and primary screening test in TB patients.
Materials and Methods
Study Design and Period
This was a retrospective cross-sectional study, conducted in the patients. Duration of the study was from June 2018 to May 2019, to evaluate drug resistance in patients attending Government Hospital of Thoracic Medicine at Chennai, Tamil Nadu, India.
Study Population
Data for 8140 patients were retrieved for a period of June 2018 to May 2019, all data related to testing and diagnosis of TB using GeneXpert MTB/RIF Assay were collected at Central laboratory, Government Hospital of Thoracic Medicine, Tambaram Sanatorium and were documented. The study was conducted in the age groups between 6-75 years with symptoms and signs suggestive of TB like fever, cough with expectoration, haemoptysis, weight loss, fatigue and with radiological symptoms suggestive of TB like cavity, consolidation etc., and lymphadenitis. Since Govt. Hospital of thoracic Medicine is a Nodal DR TB Centre and Center of Excellence for HIV/AIDS, the patients were from all over Tamil Nadu and borderline states.
Inclusion criteria
1) Based on clinical criteria: a) All presumptive TB patients- People living with HIV/AIDS (PLHIV) and all new symptomatic patients; b) All presumptive MDR patients- New patients at the time of diagnosis (Including history of contact with known MDR patients)- Previously treated patients (Relapse, failure, defaulters)- In drug sensitive patients any follow-up smear positive, non-responders and others; 2) Based on Microbiological criteria: All smear positive patients before starting Anti Tuberculosis Treatment (ATT)- Smear negative patients if signs and symptoms suggestive of TB infection.
Exclusion criteria: Stool, Blood samples, patients being treated with ATT, Monitoring bacteriological cure, Monitoring response to treatment.
Methodology
Two sputum samples, one spot and next day early morning sputum sample (A and B) collected from patients. The Extra pulmonary samples were collected by the physicians depending on the site involved. Smear was prepared from both sputum and extra pulmonary samples (pleural fluid, ascitic fluid, cerebrospinal fluid, tissue biopsy and pus) under strict aseptic precautions stained by Auramine fluorescence technique and observed under LED fluorescent microscopy for Acid Fast Bacilli (AFB). All smear positive and smear negative samples were tested for CBNAAT as per physician’s request. For GeneXpert the 2-3 mL samples are collected in two 50 mL falcon tube (one more sample as a back up for further testing). The samples were transported to the laboratory in the same day and stored in a refrigerator at 2-8°C. Both sputum and extrapulmonary samples were processed and tested as per the Cepheid MTB/RIF assay procedure guidelines [2]. The samples were processed under strict aseptic precautions in the Biosafety Cabinet Class II A2. To the collected sample, buffer added in 2:1 ratio, vortex done for few seconds and incubated at room temperature for 10 minutes, 2 mL of liquefied supernatant taken by sterile transfer pipette and transferred to the GeneXpert cartridge containing in built primers, probes, PCR reagents, barcode scanned and loaded in the GeneXpert machine. Ultrasonic lysis of the sample releases DNA and mixes with PCR reagents. Automated RT-PCR amplifies the DNA and RIF resistance detected by probes A to E specific to identify the mutant codons 507 to 533 of RRDR region of rpoB gene and the printed reports were available within two hours. The reports generated were informed to the concerned physicians and the State Treatment Laboratory Supervisor (STLS). The samples were further tested in Line Probe Assay (LPA) [8] at NIRT, Chennai for confirmation of RIF resistance and other associated resistance to isoniazid and other second line drug such as fluroquinolones and second line injectables.
Statistical Analysis
All statistical analysis were performed using SPSS version 15.0. Continuous variables were presented as mean±standard deviation or median and Interquartile Range (IQR) values, as appropriate based on the variable normal distribution. Categorical data were presented as frequencies and percentages.
Results
A total of 8140 samples were tested for CB-NAAT which include 7110 sputum samples and 1030 extra pulmonary samples. MTB detected in 3726 and RIF sensitive found in 3554 samples include 238 extra pulmonary samples and RIF resistance was detected in 172 which include 22 extra pulmonary samples [Table/Fig-1].
Flow chart for the sample distribution.
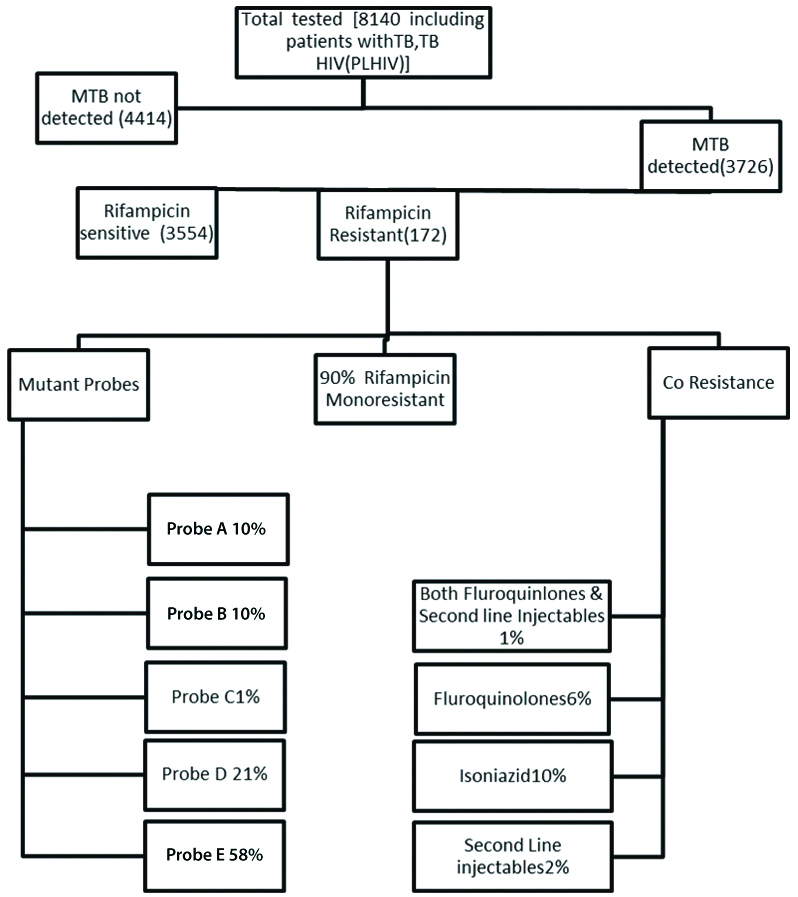
The overall sensitivity of CB-NAAT in smear negative pulmonary and extra pulmonary samples was 32%. Out of the total 6070 males, 2943 were RIF sensitive and 129 were RIF résistance. Among the 2070 females 611 were sensitive to RIF and 43 were resistant [Table/Fig-2]. Among the RIF sensitive patients, isoniazid monoresistance was found in 1.9%. The type of clinical samples used in the study and tested with CBNAAT is shown in [Table/Fig-3]. Among the TB/HIV co-infected patients 1% found to be resistance to RIF. The sensitivity of GeneXpert in smear negative sputum samples was 89% and extra pulmonary samples were 22% compared with AFB smear. In present study, 97% of the RIF resistance patients were previously treated and there is no gender preponderance as male and females are found equally susceptibility in these study. Among the 172 RIF resistance samples majority were due to mutant codon 531 of RRDR region of the rpoB gene [Table/Fig-4,5]. RIF monoresistance was found in 90% of patients which was confirmed by LPA. A 10% had isoniazid resistance 6% had fluoroquinolone resistance and 2% has resistance to Second line injectables and 1% had resistance to both. Among the 105 paediatric patients tested 10% of the patients were found MTB detected RIF sensitive [Table/Fig-6].
| Total patients tested for CBNAAT | 8140 (new-2074, Previously treated-6066) |
|---|
| Male | 6070 (75%) |
| Female | 2070 (25%) |
Source of sample tested using CB-NAAT.
| Type of clinical samples | Total samples tested | MTB not detected | MTB detected and RIF sensitive | MTB detected and RIF resistant |
|---|
| Sputum | 7110 | 3647 | 3316 | 150 |
| Pleural fluid | 414 | 332 | 78 | 3 |
| FNAC | 146 | 84 | 55 | 6 |
| Lymph node biopsy | 12 | 9 | 2 | 1 |
| Bronchoalveolar Lavage (BAL) | 300 | 239 | 55 | 5 |
| Cerebrospinal Fluid (CSF) | 41 | 36 | 5 | - |
| Ascitic fluid | 28 | 24 | 4 | - |
| Pus | 79 | 40 | 35 | 4 |
| Gastric juice | 10 | 3 | 4 | 3 |
| Total | 8140 | 4414 | 3554 | 172 |
MTB status among the population.
| MTB not detected |
| Total | 4414 |
| Male | 2998 (67.9%) |
| Female | 1416 (32.07%) |
| MTB detected and RIF sensitive | |
| Total | 3554 |
| Male | 2943 (83%) |
| Female | 611 (17%) |
| MTB detected and RIF resistant | |
| Total | 172 |
| Male | 129 (75%) |
| Female | 43 (25%) |
| HIV and TB Co infected | |
| Total | 1113 | Male | Female | Children |
| MTB detected and RIF sensitive | 159 (14%) | 80 | 49 | 30 |
| MTB detected and RIF resistant | 12 (1%) | 9 | 3 | - |
| MTB not detected | 942 | 516 | 421 | 5 |
Mutant codons detected n=172.
| Probes (Codon) | Percentage |
|---|
| Probe E (531) | 58% |
| Probe D (526) | 21% |
| Probe B (516) | 10% |
| Probe A (507) | 10% |
| Probe C (521) | 1% |
MTB paediatric patients status.
| Total | 105 |
| MTB detected and RIF sensitive | 10 (10%) |
| MTB detected and RIF resistant | - |
| Source of sample tested |
| Sample | Smear negative | MTB detected in GeneXpert |
| Sputum | 681 | 603 |
| Extrapulmonary | 981 | 211 |
Discussion
This study shows GeneXpert has more diagnostic accuracy in detecting RIF Resistance and done as a primary screening test for MTB detection in PLHIV patients who remain smear negative due to lack of caseous necrosis and cavitation, supported by study conducted at Delhi and Uttar pradesh [9,10]. GeneXpert is also more advantageous in paediatric TB whose samples like gastric aspirates remain paucibacilliary, supported by study conducted at Mangalore [11-13]. GeneXpert has a sensitivity of 32% in smear negative samples, lower sensitivity because bacillary count lower than to be detected by CB-NAAT especially in paucibacilliary extra pulmonary samples is the reason, this supported by study conducted at Italy [14] and Kolkata [15]. Performace of GeneXpert MTB/RIF from previous studies is represented in [Table/Fig-7]. In RIF sensitive 3554 patients, 10 had monoresistance to Isoniazid detected by LPA done at NIRT [5,7,9,12,14-19]. RIF resistance was detected in 172 samples, among which 97% are previously, treated patients and 3% were new patients. This is supported by the study conducted by Asian pacific society of respirology [16]. The emergence of resistant bacilli in previously treated patients was due to the wide spread use of drug, poor compliance, inappropriate and non-adherence to treatment. The aerobic resistant bacillus remains dormant inside the anaerobic environment of granulomas and cavities. Whenever this cavities or granulomas breaks down, bacilli starts multiplying in the aerobic environment and spreads via blood to other organs and via bronchus to others through aerosols. There is no gender preponderance in RIF resistance and both male and female are equally susceptible, this supported by study conducted at Zambia [17]. Probe E mutation (58%) of the rpoB gene, which denotes mutation of the 531 codon was found in majority of patients followed by probe D (21%), probe A (10%), probe B (10%) and probe C (1%), this supported by the study conducted at Malawi, Africa [7]. RIF mono resistance was detected in more than 80% of patients which was confirmed by LPA, this result is similar to the studies conducted in South Africa [18,19]. Isoniazid resistance occurs due to mutations at multiple genes including katG (catalase-peroxidase gene), inhA region (NADH-dependent-ACP-reductase In hA gene) kasA (β-keto-acyl-ACP-synthase gene), ndh (NADH-dehydrogenase) and a hpC region (alkyl-hydroxyperoxide-reductase) genes [12]. Recently available molecular methods doesn’t cover all the multiple mutations of the Isoniazid resistant genes, could be reason for the lesser detection of isoniazid co-resistance. A 6% patients has fluroquinolones resistance, 2% had resistance to second injectable like kanamycin, capreomycin and amikacin and 1% of patients had resistance to both fluroquinolones and Second line injectables as many patients sample culture results turn negative during MDR-TB treatment this is similar to the study conducted at Lesotho hospital [5]. So, early detection of RIF resistance by GeneXpert as a surrogate marker of MDR TB is necessary to reduce morbidity and mortality and to prevent transmission of MDR strains.
GeneXpert MTB/RIF from previous studies available [5,7,9,12,14-19].
| Reference | Observation |
|---|
| Dewan R et al., [9] | GeneXpert has more diagnostic accuracyIn smear negative samples (84.04% sensitivity and 80.57% specificity). |
| Anshu K et al., [12] | More advantage in paediatric (children) (92.7% sensitivity, 98.9% specificity). |
| Singh A et al., Ahmad Z and Zubair I [14,15] | Lower sensitivity in extrapulmonary samples correlates with present study. |
| Masenga SK et al., [16] | Rifampicin resistance found more in previously treated patients (5.9%). |
| Barnard M., [17] | Male and female patients are equally susceptible for rifampicin resistance. |
| Coovadia YM et al., [18], Tripathi R et al., [19] | Rifampicin monoresistance were more common 8.8%25.4%. |
| Zaw MT et al [5] | Resistance to second line drugs less detected by LPA as many patients cured (culture negative) after starting MDR treatment for rifampicin resistance. |
| Reddy R and Alvarez Uria G [7] | Similar trend were seen Probe E mutation most common (63.6%). |
Limitation(s)
Technical: GeneXpert MTB/RIF Assay requires continuous electrical supply and back up either by UPS or Genset is needed as prolonged power failure affect processing of reports and may need repeat testing. Room temperature should be maintained between 24°C to 30°C. The catridges should be stored at 2-8°C as this temperature is needed to maintain the stability of PCR reagents and buffers contained in the catridge.
Diagnostic: GeneXpert MTB/RIF Assay cannot detect RIF resistance due to mutations occurring outside the RRDR and also doesn’t cover Isoniazid coresistance. Positive reports do not indicate about the viability of the bacilli and negative report does not exclude positivity when the bacillary count is too low to be detected by the machine.
Conclusion(s)
The present study showed high prevalence of RIF resistance in previously treated patients due to the following reasons: a) Presence of previous history of ATT with poor adherence invariably leads to RIF Resistance; b) Drug sensitive patients might turn resistant as a result of mutation due to poor adherence and compliance or inappropriate regimen; c) Drug sensitive patients might turn resistant due to amplification of dormant resistant bacilli during treatment. So, all suspicion, new and previously treated patients samples should be subjected to GeneXpert MTB/RIF Assay before starting DOTS regimen as per universal DST to reduce morbidity and mortality and to prevent transmission of drug resistant strains. This study confirms that Genexpert remains the rapid diagnostic tool for the diagnosis of TB as well as to confirm sensitivity/resistance to RIF both in pulmonary and extra pulmonary samples.
Author Declaration:
Financial or Other Competing Interests: None
Was Ethics Committee Approval obtained for this study? Yes
Was informed consent obtained from the subjects involved in the study? Yes
For any images presented appropriate consent has been obtained from the subjects. NA
Plagiarism Checking Methods: [Jain H et al.]
Plagiarism X-checker: Apr 20, 2020
Manual Googling: Jul 22, 2020
iThenticate Software: Aug 29, 2020 (10%)
[1]. World Health Organization. 2020. Global Tuberculosis Report. [online] Available at: <https://www.who.int/tb/publications/global_report/en/> [Accessed 17 July 2020] [Google Scholar]
[2]. Who.int. 2020. WHO | Xpert MTB/RIF Implementation Manual. [online] Available at: <https://www.who.int/tb/publications/xpert_implem_manual/en/> [Accessed 17 July 2020] [Google Scholar]
[3]. Sachdeva KS, Kumar A, Dewan P, Kumar A, Satyanarayana S, New vision for Revised National Tuberculosis Control Programme (RNTCP): Universal access-“reaching the un-reached”Indian J Med Res 2012 135(5):690-94. [Google Scholar]
[4]. Kaur R, Jindal N, Arora S, Kataria S, Epidemiology of rifampicin resistant tuberculosis and common mutations in rpoB gene of mycobacterium tuberculosis: A retrospective study from six Districts of Punjab (India) using Xpert MTB/RIF assayJ Lab Physicians 2016 8(2):96-100.10.4103/0974-2727.18078927365918 [Google Scholar] [CrossRef] [PubMed]
[5]. Zaw MT, Emran NA, Lin Z, Mutations inside rifampicin-resistance determining region of rpoB gene associated with rifampicin-resistance in Mycobacterium tuberculosisJ Infect Public Health 2018 11(5):605-10.10.1016/j.jiph.2018.04.00529706316 [Google Scholar] [CrossRef] [PubMed]
[6]. Sharma SK, Kohli M, Yadav RN, Chaubey J, Bhasin D, Sreenivas V, Evaluating the diagnostic accuracy of Xpert MTB/RIF assay in pulmonary tuberculosisPLoS One 2015 10(10):e014101110.1371/journal.pone.014101126496123 [Google Scholar] [CrossRef] [PubMed]
[7]. Reddy R, Alvarez-Uria G, Molecular epidemiology of rifampicin resistance in mycobacterium tuberculosis using the GeneXpert MTB/RIF assay from a rural setting in IndiaJ Pathog 2017 2017:673809510.1155/2017/673809529225973 [Google Scholar] [CrossRef] [PubMed]
[8]. Nathavitharana RR, Cudahy PGT, Schumacher SG, Steingart KR, Pai M, Denkinger CM, Accuracy of line probe assays for the diagnosis of pulmonary and multidrug-resistant tuberculosis: A systematic review and meta-analysisEur Respir J 2017 49:160107510.1183/13993003.01075-201628100546 [Google Scholar] [CrossRef] [PubMed]
[9]. Dewan R, Anuradha S, Khanna A, Garg S, Singla S, Ish P, Agarwal S, Role of cartridge-based nucleic acid amplification test (CBNAAT) for early diagnosis of pulmonary tuberculosis in HIVJ Indian Acad Clin Med 2015 16:114-17. [Google Scholar]
[10]. Sahana KS, Prabhu AS, Saldanha PR, Usage of cartridge based nucleic acid amplification test (CB-NAAT/GeneXpert) test as diagnostic modality for pediatric tuberculosis; case series from Mangalore, South IndiaJ Clin Tuberc Other Mycobact Dis 2018 11:07-09.10.1016/j.jctube.2017.12.00231720384 [Google Scholar] [CrossRef] [PubMed]
[11]. Isakova J, Sovkhozova N, Vinnikov D, Goncharova Z, Talaibekova E, Aldasheva N, Mutations of rpoB, katG, inhA and ahp genes in rifampicin and isoniazid-resistant Mycobacterium tuberculosis in Kyrgyz RepublicBMC Microbiol 2018 18(1):2210.1186/s12866-018-1168-x29566660 [Google Scholar] [CrossRef] [PubMed]
[12]. Anshu K, Das S, Paul DK, A study on the role of cartridge based nucleic acid amplification test (CBNAAT) for diagnosing pediatric tuberculosis in a tertiary care hospital in Eastern IndiaAcad J Ped Neonatol 2018 6(3):55574510.19080/AJPN.2018.06.555745 [Google Scholar] [CrossRef]
[13]. Lombardi G, Di Gregori V, Girometti N, Tadolini M, Bisognin F, Dal Monte P, Diagnosis of smear-negative tuberculosis is greatly improved by Xpert MTB/RIFPLoS One 2017 12(4):e017618610.1371/journal.pone.017618628430807 [Google Scholar] [CrossRef] [PubMed]
[14]. Singh A, Karmakar D, Jha R, Comparative evaluation of cbnaat with smear microscopy, symptom screen and chest x-ray for diagnosis of pulmonary tuberculosisInt J Sci 2019 8(4):5 [Google Scholar]
[15]. Ahmad Z, Zubair I, Role of Cabnaat in the early detection of Rifampicin resistance in retreatmet cases of pulmonary and extrapulmonary tuberculosisRespirology 2017 WILEY 111 RIVER ST, HOBOKEN 07030-5774, NJ USA [Google Scholar]
[16]. Masenga SK, Mubila H, Hamooya BM, Rifampicin resistance in mycobacterium tuberculosis patients using GeneXpert at Livingstone Central Hospital for the year 2015: A cross sectional explorative studyBMC Infect Dis 2017 17(1):64010.1186/s12879-017-2750-928938874 [Google Scholar] [CrossRef] [PubMed]
[17]. Barnard M, Albert H, Coetzee G, O’Brien R, Bosman ME, Rapid molecular screening for multidrug-resistant tuberculosis in a high-volume public health laboratory in South AfricaAm J Respir Crit Care Med 2008 177(7):787-92.10.1164/rccm.200709-1436OC18202343 [Google Scholar] [CrossRef] [PubMed]
[18]. Coovadia YM, Mahomed S, Pillay M, Werner L, Mlisana K, Rifampicin mono-resistance in Mycobacterium tuberculosis in KwaZulu-Natal, South Africa: A significant phenomenon in a high prevalence TB-HIV regionPLoS One 2013 8(11):e7771210.1371/journal.pone.007771224223122 [Google Scholar] [CrossRef] [PubMed]
[19]. Tripathi R, Kashyap S, Chaubey P, Pandey AP, Anupurba S, Role of cartridge-based nucleic acid amplification test in detection of pulmonary tuberculosis in people living with human immunodeficiency virusJournal of The Academy of Clinical Microbiologists 2017 19(2):11410.4103/jacm.jacm_37_16 [Google Scholar] [CrossRef]