Predictive Value of the New Inflammatory Score Based on C-reactive Protein to Albumin Ratio in Serious Postoperative Complications after Curative Pancreaticoduodenectomy for Pancreatic Adenocarcinoma
Ümit Mercan1, Ogün Erşen2, Cemil Yüksel3, Ali Ekrem Ünal4
1 Lecture, Department of General Surgery, Surgical Oncology, Ankara University Medicine Faculty, Ankara, Turkey.
2 Lecture, Department of General Surgery, Surgical Oncology, Ankara University Medicine Faculty, Ankara, Turkey.
3 Faculty, Department of General Surgery, Surgical Oncology, Ankara University Medicine Faculty, Ankara, Turkey.
4 Professor, Department of General Surgery, Surgical Oncology, Ankara University Medicine Faculty, Ankara, Turkey.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Ümit Mercan, Ankara University Medicine Faculty, Surgical Oncology Clinic, Ankara, Turkey.
E-mail: Umit.mercan@yahoo.com.tr
Introduction
The CRP/Albumin Ratio (CAR) which has been shown to be closely related to the results in septic patients has been found to be also associated with postoperative and long-term outcomes in patients with malignant diagnosis. Although the prognostic value of CAR in many gastrointestinal cancers has been determined, studies on pancreatic cancer are limited.
Aim
To evaluate the predictive value of CAR on serious postoperative complications after curative pancreaticoduodenectomy for pancreatic cancer.
Materials and Methods
One hundred eighty seven patients that underwent curative resection for pancreatic cancer between January 2010 and January 2020 were included in the study. The optimal cut-off level of the CAR was calculated as 0.22. Patients were divided into two groups as below and above this value. Clinicopathological and postoperative variables were compared between the groups.
Results
In univariate and multivariate analysis, modified Glasgow Prognostic Score (mGPS) (OR: 3.67. %95 CI: 2.31~5.82. p=0.028), CAR (OR: 8.02 %95 CI: 3.45~21.62. p>0.001), lymph node status (OR: 1.54. %95 CI: 0.41~3.39. p=0.042) and TNM stage (OR: 3.92. %95 CI: 1.56~10.08. p= 0.005) were found to be independent risk factors for serious postoperative complications.
Conclusion
Based on these data, CAR, which can be easily measured from preoperative biochemical results in each patient, may be an independent and significant predictor of postoperative serious complications and poor outcomes after pancreaticoduodenectomy in patients with pancreatic cancer.
Gastrointestinal cancers, Inflammation, Surgery
Introduction
Although the frequency of pancreatic cancer is lower than gastric and colon cancers, it is a type of cancer that is diagnosed late due to its insidious course and the symptoms are mild and continues to remain low and have high curability [1]. Despite the improvement in medical treatments and increasing success in surgical results, five year survival rates do not exceed 35% [2,3]. It has been determined that many patients and tumour-related prognostic factors are closely related to postoperative and long-term results [4]. While the effects of tumour size, local invasion degree of the tumour, lymph node involvement rate, and tumour relationship conditions such as vascular or neural invasion on the results are well known, in recent studies it has been proven that systemic inflammatory response as a patient-related factor plays an important role in tumour progression and postoperative results [4,5].
Systemic inflammatory response, which can be demonstrated by parameters such as the Neutrophil to Lymphocyte Ratio (NLR) and modified Glasgow Pognostic Score (mGPS) has been reported to be a potent predictor of postoperative outcomes and cancer-specific survival [6,7]. The mGPS is determined by serum C-Reactive Protein (CRP) and albumin levels and has been shown to have prognostic significance in septic and cancer patients [8,9]. In addition, the CAR, a new inflammation-based score, which has been shown to be closely related to the results in many studies conducted in septic patients, has also been associated with postoperative and long-term outcomes in patients with malignant diagnosis [8,9]. Although the prognostic value of CAR in patients with various gastrointestinal cancers has been studied, studies on pancreatic cancer patients are limited [10,11]. In this study, the relationship between preoperative CAR and severe postoperative complications has been investigated retrospectively in patients undergoing curative pancreaticoduodenectomy for pancreatic cancer.
Materials and Methods
Patient Selection and Data Collection
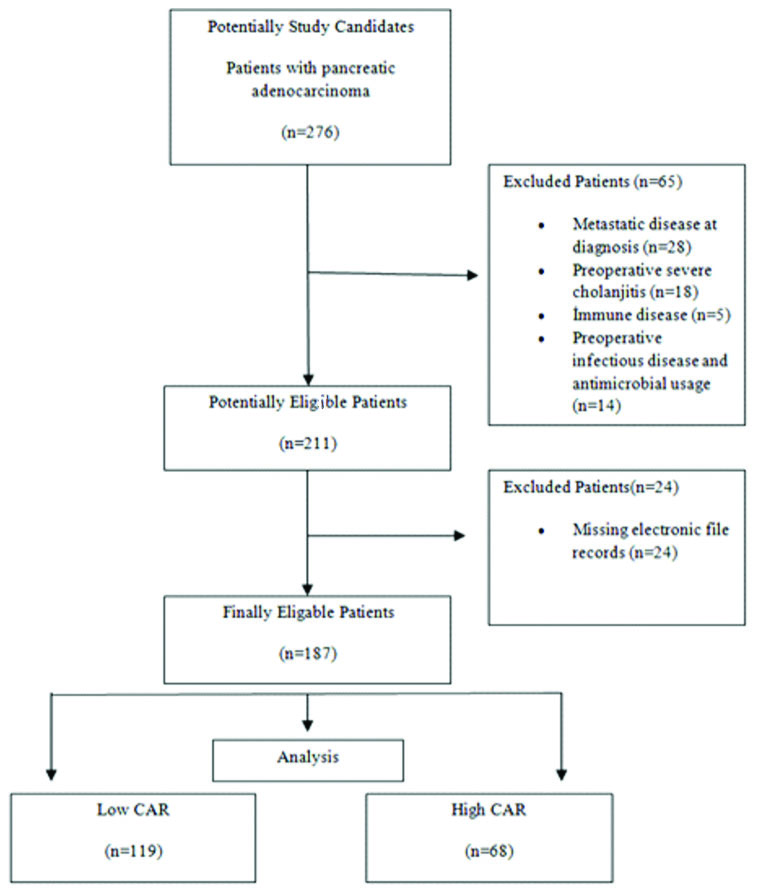
Between January 2010 and January 2020, 187 patients who underwent curative pancreaticoduodenectomy for pancreatic cancer were retrospectively analysed. The patients with missing electronic file records, having metastatic disease at the time of diagnosis, severe cholangitis attack in preoperative period, having immune or any systemic disease in which inflammatory processes were intense and those that were on antibiotic treatment were excluded from the study. Permission for the use of patient data was obtained from the Administiration of Ankara University Cebeci Hospital {15255985-622.03(774.99)-E.31084}.
Preoperative haemogram and biochemistry values were recorded. The level of systemic inflammatory response in each patient was determined by mGPS and CAR values obtained using preoperative serum CRP and albumin levels. The mGPS was calculated on the basis of known blood CRP and albumin levels [9]. The albumin values were divided into two groups ≥35 g/L and ≤35 g/L. Then, CRP values were divided into two groups as ≥10 mg and <10 mg. Patients with normal albumin (≥35 g/L) and patients with normal CRP level (≤10 mg/L) were considered as mGPS 0, patients with normal albumin and high CRP levels (>10 mg) were considered as mGPS 1 and patients with low albumin (<35 g/L) and high CRP level (>10 mg/L) were considered as mGPS 2. The CAR was obtained by dividing the serum CRP level (mg/L) by the serum albumin level (g/L). The optimal cut-off level of the CAR was calculated as 0.22 (AUC: 0.852±0.03. %95 CI: 0.779~0.924. p=0.001) with 81% sensitivity and 78% specificity in Receiver Operating Curve (ROC) analysis and patients were divided into two groups as below and above of this level.
A total of 187 patients data were recorded including age, gender, BMI, ASA score, comorbid diseases, weight loss percentage, T stage, lymph node status, TNM stage, intraoperative blood transfusion requirement, duration of operation, Clavien-Dindo grade 3 or higher postoperative complications, pancreatic fistula, mGPS, CAR, length of hospital stay, length of stay in intensive care unit and mortality rate. The pathology specimens of the patients diagnosed with pancreatic cancer were retrospectively analysed through the hospital database. Tumour and lymph node status of patients were determined according to AJCC, Pancreas Cancer Staging 8th Edition [12]. Postoperative complications were determined based on the Modified Clavien Dindo Classification and the presence of grade 3 or higher complications were evaluated [13]. Pancreatic fistulas were categorised using the International Pancreatic Fistula Study definitions [14]. The relationship between CAR and postoperative serious complication rates were investigated [Table/Fig-1].
Flowchart of total patient enrollment.
Statistical Analysis
Numerical data were expressed as mean±Standard Deviation (SD). X2 test, Student T-test, Mann-Whitney U-test were used for the relationship between numerical and categorical data. The optimal cut-off level of CAR was determined by ROC analysis. Binary logistic regression analysis was used to determine the factors affecting postoperative complications. All p-values less than 0.05 were considered statistically significant. These analyses were performed using IBM SPSS statistical version 23.0.
Results
The clinicopathological data is summarised in [Table/Fig-2] as mean±SD, range or ratio [12]. The mean age of 187 patients included in the study was 60.17±7 years and 119 (63.6%) patients were male. Preoperative high CAR (>0.22) was seen in 68 (36.4%) patients. There was no significant difference between the groups in terms of age, sex, comorbid diseases and BMI. Although not statistically significant, ASA 3 patients had a higher number in the group of high CAR (p=0.055). There was a significant difference between the groups in terms of weight loss percentage, T stage, lymph node status, TNM stage and mGPS score. The relationship between postoperative outcomes and high and low CAR groups are summarised in [Table/Fig-3] [14]. There was no significant difference between the groups in terms of operation time, intraoperative blood transfusion, length of hospital stay and pancreatic fistula rates. The length of stay in the intensive care unit was significantly higher in the high CAR group than the other group (p=0.022). Similarly, Clavien-Dindo grade 3 and serious complications and mortality rates were significantly higher in high CAR group compared to the other group.
Relationship between clinicopathological variables and high and low CAR groups.
| Variable | Total (n=187) | CAR:High (>0.22) (n=68) | CAR:Low (<0.22) (n=119) | p-value |
|---|
| Age (years) | 60.17±7.7 | 60.24±7.37 | 60±8.03 | 0.932 |
| Gender (male) | 119 (63.6) | 41 (60.3) | 78 (65.5) | 0.287 |
| BMI (kg/m2) | 26.57±4.16 | 26.96±4.57 | 26.35±3.9 | 0.337 |
| ASA score |
| 1 | 17 (9.1) | 5 (7.4) | 12 (10.1) | 0.055 |
| 2 | 79 (42.2) | 22 (32.4) | 57 (47.9) |
| 3 | 91 (48.7) | 41(60.3) | 50 (42) |
| Comorbidity |
| DM | 55 (29.4) | 22 (32.4) | 33 (27.7) | 0.510 |
| HT | 87 (46.5) | 37 (54.4) | 50 (42) | 0.069 |
| COLD | 26 (13.9) | 12 (17.6) | 14 (11.8) | 0.279 |
| Weight loss (%) | 3.96±5.13 | 6.59±6.01 | 2.46±3.83 | >0.001 |
| T stage |
| T1 | 6 (3.2) | 0 (0) | 6 (5) | 0.002 |
| T2 | 45 (24.1) | 11 (16.2) | 34 (28.6) |
| T3 | 115 (61.5) | 43 (63.2) | 72 (60.5) |
| T4 | 21 (11.2) | 14 (20.6) | 7 (5.9) |
| Lymph node status |
| N0 | 102 (54.5) | 21 (30.9) | 81 (68.1) | >0.001 |
| N1 | 67 (35.8) | 35 (51.5) | 32 (26.9) |
| N2 | 18 (9.6) | 12 (17.6) | 6 (5) |
| TNM stage* |
| Stage 1 | 32 (17.1) | 3 (4.4) | 29 (24.4) | >0.001 |
| Stage 2 | 120 (64.2) | 42 (61.8) | 78 (65.5) |
| Stage 3 | 35 (18.7) | 23 (33.8) | 12 (10.1) |
| mGPS |
| 0 | 133 (71.1) | 14 (20.6) | 119 (100) | >0.001 |
| 1 | 14 (7.5) | 14 (20.6) | 0 (0) |
| 2 | 40 (21.4) | 40 (58.8) | 0 (0) |
Numerical values are given as mean±standard error. CRP: C-reactive protein, BMI: Body mass index, ASA: American society of anesthesiologists, DM: Diabetes mellitus, HT: Hypertension, COLD: Chronic obstructive lung disease, TNM: Tumour node metastasis, mGPS: Modified glasgov prognostic score, *TNM stage was determined according to AJCC Pancreatic Cancer Staging 8th Edition [12]; p-value <0.05 was considered statistically significant
Relation between postoperative outcomes and high and low CAR groups.
| Variable | Total(n=187) | CAR: High(>0.22)(n=68) | CAR: Low(>0.22)(n=119) | p-value |
|---|
| Operation time (min) | 189.81±60.34 | 182.21±54.89 | 194.16±63.05 | 0.193 |
| Intraoperative blood transfusion requirement (number of patients) | 18 (9.6) | 5 (7.4) | 13 (10.9) | 0.300 |
| Hospital stay (day) | 9.41±5.98 | 10.91±8.34 | 8.55±3.85 | 0.098 |
| ICU stay (day) | 2.48±3.61 | 3.28±5.09 | 2.03±2.30 | 0.022 |
| In-hospital mortality | 7(3.7) | 3(4.4) | 4(3.3) | 0.452 |
| Postoperative complications (CDgrade 3 and above) | 42 (22.5) | 34 (50) | 8 (6.7) | <0.001 |
| Pancreatic fistula* |
| Grade A | 22 (11.8) | 12 (17.6) | 10 (8.4) | 0.510 |
| Grade B | 12 (6.4) | 7 (10.3) | 5 (4.2) |
| Grade C | 2 (1.1) | 0 (0) | 2 (1.7) |
Numerical values are given as mean±standard error. CRP: C-reactive protein, ICU: Intensive care unit, CD: Clavien-Dindo. *Pancreatic fistulas were categorised using the definitions of the International Pancreatic Fistula Working Group [14]; p-value <0.05 was considered statistically significant
Univariate and multivariate analysis results of risk factors that may affect postoperative serious complications are summarised in [Table/Fig-4]. In univariate analysis; mGPS, CRP/Albumin ratio, lymph node status and TNM stage were found statistically significant. When these data were included in the multivariate analysis, high mGPS (OR: 3.67. 95% CI: 2.31~5.82. p=0.028), high CAR (OR: 8.02. 95% CI: 3.45~21.62. p≤0.001), lymph node status (OR: 1.54. 95% CI: 0.41~3.39. p=0.042) and TNM stage (OR: 3.92. 95% CI: 1.56~10.08. p=0.005) were found as independent risk factors for severe postoperative complications.
Univariate and multivariate analysis of risk factors associated with severe postoperative complications.
| Variable | Univariate analysis | Multivariate analysis |
|---|
| OR(%95 CI) | p-value | Adjusted OR (%95 CI) | p-value |
|---|
| mGPS | 10.75(4.82~23.97) | >0.001 | 3.67(2.31~5.82) | 0.028 |
| CAR | 13.87(5.86~32.81) | >0.001 | 8.02(3.45~21.62) | <0.001 |
| Lymph node status | 3.54(1.70~7.39) | 0.008 | 1.54(0.41~3.39) | 0.042 |
| TNM stage | 4.08(1.85~8.97) | 0.023 | 3.92(1.56~10.08) | 0.005 |
OR: Odds ratio, mGPS: Modified glasgow prognostic score, CRP: C-reactive protein, TNM: Tumour-node-metastasis; p-value <0.05 was considered statistically significant
Discussion
Pancreatic cancer is a type of cancer that can be diagnosed late due to its nonspecific symptoms and insidious course and its outcomes are quite bad. Despite the improvement and experience in surgical and medical treatments, mortality rates remain high [2]. Most of patients have inoperable disease at the time of diagnosis and only 20% of cases have a chance of curative resection. Pancreatic cancer continues to be the most challenging type of cancer in practice, with its surgical procedures and operational outcomes.
Tumour-related prognostic conditions such as tumour size, local invasion, lymph node status, perineural or vascular invasion and surgical margin have been reported to be closely associated with postoperative outcomes and survival in patients with pancreatic cancer [4]. It is known that tumour-related conditions, as well as patient-related conditions, have an effect on postoperative outcomes. Nutritional factors and various comorbid conditions can be counted as patient-related factors. In addition, many recent studies have shown that systemic inflammatory response is closely associated with poor outcomes. Systemic inflammatory response, which can be demonstrated by parameters such as the mGPS or NLR has been reported to be an important predictor of many gastrointestinal cancer-specific survival [6,7].
In the present study, high mGPS, high CAR, advanced TNM stage and the degree of lymph node involvement were found to be independent risk factors for serious postoperative complications in patients with pancreatic cancer undergoing curative resection and these results show that there is a close relationship between systemic inflammatory response and postoperative and short-term results.
The CAR, which was defined for the first time in septic patients, has been found to be a potent indicator of the inflammatory process. Miyamoto T et al., have found in a study of cancer and noncancer patients that CAR is an independent factor on the prediction of postoperative short-term survival results [15]. Similar results have been achieved in many gastrointestinal cancer types. In a study reported by Ishizuka M et al., significant relationship between CAR and survival has been found in colorectal cancer patients [16]. In another study reported by Ge X et al., CAR has been found to be an independent risk factor for postoperative complications [17]. Mao M et al., have shown that CAR, NLR and the combination of these two are associated with survival in gastric cancer patients [18]. In the present study, it has been found that CAR is an independent risk factor for severe postoperative complications in patients undergoing curative pancreaticoduodenectomy for pancreatic cancer. A retrospective study of 142 pancreatic cancer patients by Arima K et al., found that postoperative CAR is associated with postoperative complications and poor survival [19]. However, only preoperative values were used in this study since surgical stress alone could affect postoperative CRP and albumin levels.
It is known that systemic inflammatory response plays a vital role in the progression of any cancer type. The CRP produced from liver is an acute-phase reactant in the inflammatory process that is up-regulated by pro-inflammatory cytokines, especially the Tumour Necrosis Factor (TNF). Extensive secretion of pro-inflammatory cytokines to peritumoural area can lead to inflammation that potentiates tumoural angiogenesis [20]. In angiogenesis, it is known that Vascular Endothelial Growth Factor (VEGF) is the most potent pro-angiogenic factor and it is secreted by tumoural cells like normal body cells but more. Many studies have found that angiogenesis is closely associated with growth, local invasion or metastasis of tumour cells as well as patient prognosis and VEGF level increases in presence of high serum CRP level [21,22]. In the lights of this information, it is considered that there is a relationship between tumour size, local invasion or distant spread and CRP levels. Similarly, in the index study, patients with advanced TNM stage were more frequent in the group with high CAR than the other.
Hypoalbuminemia is frequently seen in patients with more advanced disease and is generally considered as a potent indicator of malnutrition and cachexia. Many studies have reported that poor nutritional status has negative effect on patient survival [23-25]. As the albumin level decreases, CAR increases and high CAR may be related to the nutritional or septic status of the patient. Preoperative nutritional support is recommended to improve perioperative outcomes of patient with pancreatic cancer [26]. Further research to evaluate the relationship between immunonutrition and this inflammatory-based prognostic score is important to improve management of patients with pancreatic cancer.
Limitation(s)
Since, it was a retrospective study conducted from a single centre, possible selection bias and low sample size that may limit the applicability of these findings to other populations are the most important limitations of present study. These findings need to be supported by multicentre prospective studies in the future. Despite these limitations, we believe that our results are valuable to help improve understanding the role of systemic inflammatory response on postoperative outcomes in patients with pancreatic cancer.
Conclusion(s)
Preoperative high CAR has been found to be an independent risk factor for severe postoperative complications after curative pancreaticoduodenectomy for pancreatic cancer. By using preoperative CAR that can be easily measured by routine biochemical examination, risk stratification may be performed between the patients; essential nutritional support may be provided to required patient and also necessary precautions may be taken against potential complications and deaths in patients with high risk.
Numerical values are given as mean±standard error. CRP: C-reactive protein, BMI: Body mass index, ASA: American society of anesthesiologists, DM: Diabetes mellitus, HT: Hypertension, COLD: Chronic obstructive lung disease, TNM: Tumour node metastasis, mGPS: Modified glasgov prognostic score, *TNM stage was determined according to AJCC Pancreatic Cancer Staging 8th Edition [12]; p-value <0.05 was considered statistically significant
Numerical values are given as mean±standard error. CRP: C-reactive protein, ICU: Intensive care unit, CD: Clavien-Dindo. *Pancreatic fistulas were categorised using the definitions of the International Pancreatic Fistula Working Group [14]; p-value <0.05 was considered statistically significant
OR: Odds ratio, mGPS: Modified glasgow prognostic score, CRP: C-reactive protein, TNM: Tumour-node-metastasis; p-value <0.05 was considered statistically significant
Author Declaration:
Financial or Other Competing Interests: None
Was Ethics Committee Approval obtained for this study? Yes
Was informed consent obtained from the subjects involved in the study? NA
For any images presented appropriate consent has been obtained from the subjects. NA
Plagiarism Checking Methods: [Jain H et al.]
Plagiarism X-checker: Apr 04, 2020
Manual Googling: Apr 23, 2020
iThenticate Software: Aug 27, 2020 (19%)
[1]. Anderson KE, Mack T, Silverman D, Cancer of the pancreas. In: Schottenfeld D, Fraumeni JF edCancer epidemiology and prevention 2006 New YorkOxford University:721-62.10.1093/acprof:oso/9780195149616.003.0038 [Google Scholar] [CrossRef]
[2]. Layfield LJ, Jarboe EA, Cytopathology of the pancreas: Neoplastic and non neoplastic entitiesAnn Diagn Pathol 2010 14:140-51.10.1016/j.anndiagpath.2009.12.00720227021 [Google Scholar] [CrossRef] [PubMed]
[3]. Winter JM, Cameron JL, Campbell KA, Arnold MA, Chang DC, Coleman J, 1423 pancreaticoduodenectomies for pancreatic cancer: A single-institution experienceJournal of Gastrointestinal Surgery 2006 10(9):1199-211.10.1016/j.gassur.2006.08.01817114007 [Google Scholar] [CrossRef] [PubMed]
[4]. Jamieson NB, Denley SM, Logue J, MacKenzie DJ, Foulis AK, Dickson EJ, A prospective comparison of the prognostic value of tumour- and patient-related factors in patients undergoing potentially curative surgery for pancreatic ductal adenocarcinomaAnnals of Surgical Oncology 2011 18(8):2318-28.10.1245/s10434-011-1560-321267785 [Google Scholar] [CrossRef] [PubMed]
[5]. Pages F, Galon J, Dieu-Nosjean MC, Tartour E, Sautes-Fridman C, Immune infiltration in human tumours: A prognostic factor that should not be ignoredOncogene 2010 29(8):109310.1038/onc.2009.41619946335 [Google Scholar] [CrossRef] [PubMed]
[6]. McMillan DC, An inflammation-based prognostic score and its role in the nutrition-based management of patients with cancer: Nutrition Society and BAPEN Medical Symposium on ‘Nutrition support in cancertherapy’Proceedings of the Nutrition Society 2008 67(3):257-62.10.1017/S002966510800713118452641 [Google Scholar] [CrossRef] [PubMed]
[7]. La Torre M, Nigri G, Cavallini M, Mercantini P, Ziparo V, Ramacciato G, The glasgow prognostic score as a predictor of survival in patients with potentially resectable pancreaticadenocarcinomaAnnals of Surgical Oncology 2012 19(9):2917-23.10.1245/s10434-012-2348-922488099 [Google Scholar] [CrossRef] [PubMed]
[8]. Ranzani OT, Zampieri FG, Forte DN, Azevedo LCP, Park M, C-reactive protein/albumin ratio predicts 90-day mortality of septicpatientsPloS one 2013 8(3):e5932110.1371/journal.pone.005932123555017 [Google Scholar] [CrossRef] [PubMed]
[9]. Proctor MJ, Morrison DS, Talwar D, Balmer SM, Fletcher CD, O’Reilly DSJ, A comparison of inflammation-based prognostic scores in patients with cancer. A Glasgow Inflammation Outcome StudyEuropean Journal of Cancer 2011 47(17):2633-41.10.1016/j.ejca.2011.03.02821724383 [Google Scholar] [CrossRef] [PubMed]
[10]. Fan Z, Luo G, Gong Y, Xu H, Qian Y, Deng S, Prognostic value of the C-Reactive Protein/Lymphocyte ratio in pancreatic cancerAnnals of Surgical Oncology 2020 :01-09. [Google Scholar]
[11]. Murakawa M, Yamamoto N, Kamioka Y, Kamiya M, Kobayashi S, Ueno M, Clinical implication of pre-operative c-reactive protein-albumin ratio as a prognostic factor of patients with pancreatic ductal adenocarcinoma: A single-institutional retrospective studyIn Vivo 2020 34(1):347-53.10.21873/invivo.1178031882498 [Google Scholar] [CrossRef] [PubMed]
[12]. Chun YS, Pawlik TM, Vauthey JN, 8th Edition of the AJCC Cancer Staging Manual: Pancreas and Hepatobiliary CancersAnn Surg One 2018 25(4):845-47.10.1245/s10434-017-6025-x28752469 [Google Scholar] [CrossRef] [PubMed]
[13]. Dindo D, Demartines N, Clavien PA, Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a surveyAnnals of Surgery 2004 240(2):20510.1097/01.sla.0000133083.54934.ae15273542 [Google Scholar] [CrossRef] [PubMed]
[14]. Dusch N, Lietzmann A, Barthels F, Niedergethmann M, Rückert F, Wilhelm TJ, International study group of pancreatic surgery definitions for post pancreatectomy complications: Applicability at a high-volume centerScandinavian Journal of Surgery 2017 106(3):216-23.10.1177/145749691668094428376656 [Google Scholar] [CrossRef] [PubMed]
[15]. Miyamoto T, Fujitani M, Fukuyama H, Hatanaka S, Koizumi Y, Kawabata A, The C-Reactive Protein/Albumin Ratio is useful for predicting short-term survival in cancer and noncancer patientsJournal of Palliative Medicine 2019 22(5):532-37.10.1089/jpm.2018.040430570426 [Google Scholar] [CrossRef] [PubMed]
[16]. Ishizuka M, Nagata H, Takagi K, Iwasaki Y, Shibuya N, Kubota K, Clinical significance of the C-reactive protein to albumin ratio for survival after surgery for colorectal cancerAnnals of Surgical Oncology 2016 23(3):900-07.10.1245/s10434-015-4948-726530445 [Google Scholar] [CrossRef] [PubMed]
[17]. Ge X, Cao Y, Wang H, Ding C, Tian H, Zhang X, Diagnostic accuracy of the postoperative ratio of C-reactive protein to albumin for complications after colorectal surgeryWorld Journal of Surgical Oncology 2017 15(1):1510.1186/s12957-016-1092-128069031 [Google Scholar] [CrossRef] [PubMed]
[18]. Mao M, Wei X, Sheng H, Chi P, Liu Y, Huang X, C-reactive protein/albumin and neutrophil/lymphocyteratios and their combination predict overall survival in patients with gastric cancerOncology letters 2017 14(6):7417-24.10.3892/ol.2017.7179 [Google Scholar] [CrossRef]
[19]. Arima K, Yamashita YI, Hashimoto D, Nakagawa S, Umezaki N, Yamao T, Clinical usefulness of postoperative C-reactive protein/albumin ratio in pancreatic ductal adenocarcinomaThe American Journal of Surgery 2018 216(1):111-15.10.1016/j.amjsurg.2017.08.01628859917 [Google Scholar] [CrossRef] [PubMed]
[20]. Chen HH, Chen IH, Liao CT, Wei FC, Lee LY, Huang SF, Preoperative circulating Creactive protein levels predict pathological aggressiveness in oral squamous cell carcinoma: A retrospective clinicalstudyClinical Otolaryngology 2011 36(2):147-53.10.1111/j.1749-4486.2011.02274.x21332670 [Google Scholar] [CrossRef] [PubMed]
[21]. Li Y, Zhai Z, Liu D, Zhong X, Meng X, Yang Q, CD105 promotes hepatocarcinoma cell invasion and metastasis through VEGFTumour Biol 2015 36:737-45.10.1007/s13277-014-2686-225286761 [Google Scholar] [CrossRef] [PubMed]
[22]. Poon RTP, Fan ST, Wong J, Clinical significance of angiogenesis in gastrointestinal cancers: A target for novel prognostic and therapeutic approachesAnnals of Surgery 2003 238(1):910.1097/01.sla.0000075047.47175.3512832961 [Google Scholar] [CrossRef] [PubMed]
[23]. McMillan DC, Systemic inflammation, nutritional status and survival in patients with cancerCurrent Opinion in Clinical Nutrition and Metabolic Care 2009 12(3):223-26.10.1097/MCO.0b013e32832a790219318937 [Google Scholar] [CrossRef] [PubMed]
[24]. Oh SE, Choi MG, Seo JM, An JY, Lee JH, Sohn TS, Prognostic significance of perioperative nutritional parameters in patients with gastric cancerClinical Nutrition 2019 38(2):870-76.10.1016/j.clnu.2018.02.01529503057 [Google Scholar] [CrossRef] [PubMed]
[25]. Bicakli DH, Uslu R, Güney SC, Coker A, The Relationship Between Nutritional Status, Performance Status, and Survival Among Pancreatic Cancer PatientsNutrition and Cancer 2020 72(2):202-08.10.1080/01635581.2019.1634217 [Google Scholar] [CrossRef]
[26]. Afaneh C, Gerszberg D, Slattery E, Seres DS, Chabot JA, Kluger MD, Pancreatic cancer surgery and nutrition management: A review of the current literatureHepatobiliary Surgery and Nutrition 2015 4(1):59 [Google Scholar]