Meningiomas are tumours that originate from the arachnoid cells (meningocytes) and the majority are benign grade I tumours according to World Health Organisation (WHO). Chordoid meningioma is an uncommon variant of meningioma and corresponds to grade II tumour in WHO classification of tumours of the Nervous System 2016, because of its more aggressive behaviour and increased likelihood of recurrence. These meningiomas may appear extracranially (i.e. head and neck region, sinonasal tract, ear, temporal bone, scalp, etc) in only 2% cases. The histopathological and immunohistochemical evaluation is usually diagnostic. Here, the authors present a case of Chordoid meningioma in a 32-year-old patient who presented with complaints of nasal obstruction clinically suspected to be due to nasal polyp.
Aggressive tumour, Chordoid meningioma, Immunohistochemistry
Case Report
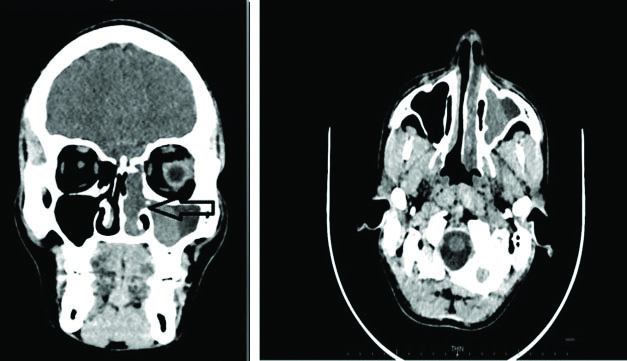
A 32-year-old male patient, presented with complaints of nasal obstruction, difficulty in breathing and 4-5 episodes of epistaxis since six months. There was no history of pain or any systemic diseases. There was no history of trauma, seizures, or loss of consciousness. On anterior Rhinoscopy, a pinkish polypoidal mass was present in left nasal cavity. On posterior Rhinoscopy, the mass was projecting in left nasopharyngeal air space and on probe test, the mass was seen arising from lateral wall of nose. On Diagnostic Nasal Endoscopy (DNE), a firm pinkish polypoidal mass was visualised in left nasal cavity, coming from middle meatus with no attachment to roof, floor and medial wall of nasal cavity. Ear and throat examination showed no abnormality; on indirect laryngoscopy, no abnormality was detected and clinically possibility of nasal angiomatous polyp was suspected. Contrast-Enhanced Computed Tomography (CECT) of the nose and paranasal sinuses revealed an ill-defined polypoidal non-enhancing soft tissue density lesion in left maxillary sinus and left ethmoidal sinus extending into the left nasal cavity involving the middle meatus causing mild deviation of nasal septum to right side. It was causing widening of ostium at medial wall of left maxillary sinus. Left nasal air space was obliterated and a provisional diagnosis of sinonasal neoplasm or fibroangiomatous polyp was made [Table/Fig-1]. All routine blood investigations were normal. Endoscopic excision of nasal mass along with Functional Endoscopic Sinus Surgery (FESS) was performed and the mass was sent for histopathological examination.
An ill-defined polypoidal non-enhancing soft tissue density lesion was noted in left maxillary sinus and left ethmoidal sinus extending into the left nasal cavity involving the middle meatus causing mild deviation of nasal septum to right side.
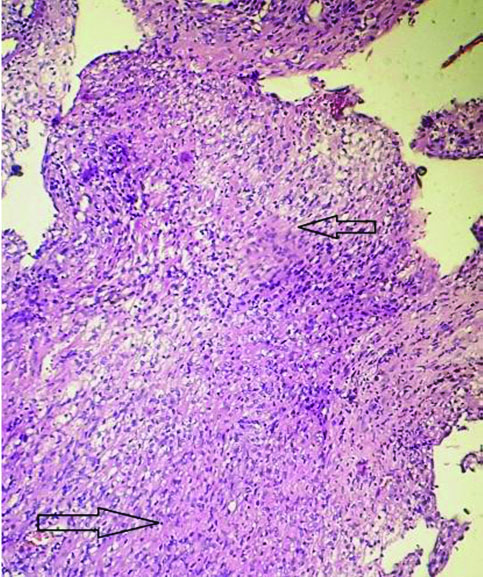
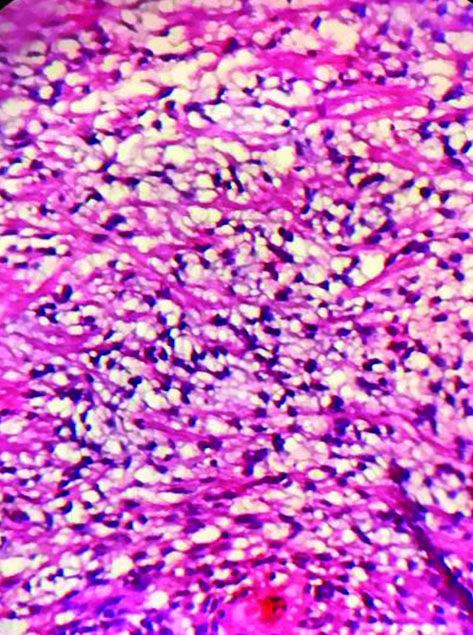
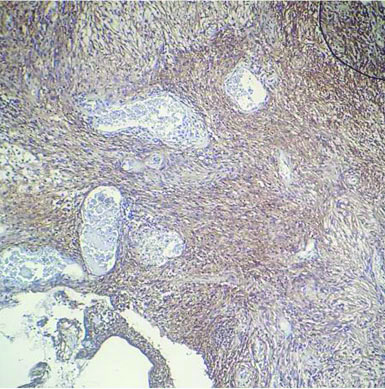
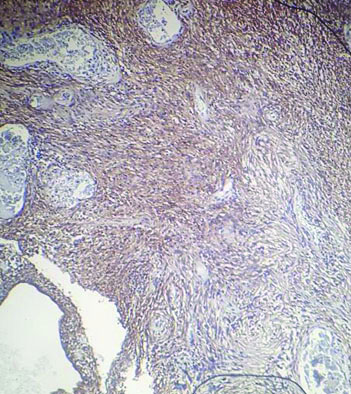
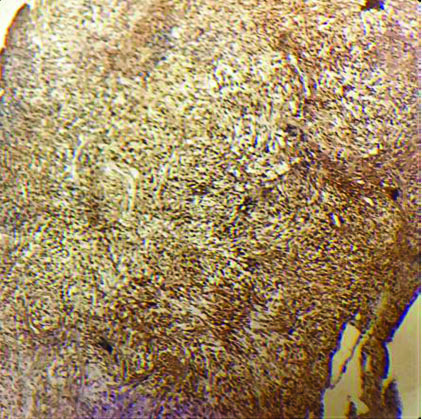
The tissue received for histopathological examination was irregular, grey-white, measuring 2×1×0.8 cm. Examination of the haematoxylin and eosin stained sections showed meningothelial cells arranged in sheets, nests and cords in a whorled pattern. The tumour cells were round to spindle shaped, displaying fused cytoplasmic membranes and indistinguishable cellular borders with myxoid and mucinous background. The nuclei were bland; and the cytoplasm of many of the tumour cells showed vacuolation reminiscent of physalipharous cells. No atypia was present and sparse mitoses were seen [Table/Fig-2,3]. Immunohistochemical staining showed positive staining of tumour cells for Epithelial Membrane Antigen (EMA), S100 and Vimentin [Table/Fig-4,5 and 6]. Tumour cells were negative for CK7, CK20 and Glial Fibrillary Acidic Protein (GFAP). Based on these findings, a final diagnosis of Chordoid meningioma (WHO Grade II) was rendered. Postoperative recovery was uneventful and no recurrence was found after 18 months follow-up.
Meningothelial cells in sheets and cells with vacuolated cytoplasm against a myxoid and delicate fibrous background. (H&E, 100x).
Microphotograph showing physalipharous-like tumour cells with intracytoplasmic vacuolisation characteristic of chordoid meningioma. (H&E, 400x). (Images from left to right)
Tumour cells showed diffuse strong positivity for EMA. (IHC 100x).
Tumour cells showed strong positivity for S-100 protein. (IHC 100x).
Tumour cells showed diffuse strong positivity for vimentin. (IHC 100x).
Discussion
Meningiomas comprise 24-30% of all intracranial neoplasms. The cell of origin is suppose to be arachnoid cap cells or meningocytes which are further derived from neural crest cells. Extra cranial meningiomas occurring in head and neck regions, sinonasal tract, ear, temporal bone, and scalp account for 2% cases of meningiomas and have very high rate of recurrence after subtotal resection [1]. A 20% of extracranial meningiomas are secondary extensions of intracranial tumours. Primary extracranial meningiomas without direct communication with the intracranial region are rare. Histologically, primary extracranial meningiomas are identical to intracranial counterparts.
Chordoid meningiomas often presents as a large supratentorial mass and headache is one of the commonest presenting complaints. The incidence ranges from 0.5% to 1% of all meningiomas and there is no age or sex predilection. This tumour is a rare variant of meningioma which consists of a myxoid pattern, cords of trabeculae and eosinophilic vacuolated cells in an abundant mucoid matrix. This chordoid area comprises the majority (about 95%) of the tumour, which is intermixed with small areas of conventional meningioma.
A prominent lymphoplasmocytic infiltrate may also be seen in some. Patients can mostly present with iron-refractory anaemia and polyclonal gammopathy (Castleman’s disease), both of which remit with resection [2]. This variant may have prognostic significance since many cases noted in the past have behaved in an aggressive manner. We present here a rare case of Sinonasal Chordoid Meningioma.
Chordoid meningioma, a variant of meningioma with aggressive behaviour and increased likelihood of recurrence [3]. Recurrences are mostly seen with incomplete excision of the tumour [4]. The entity of chordoid meningioma was first described in 1988 which earlier used to be known as myxoid/myxomatous variant of meningioma. Chordoid meningiomas are typically large, supratentorial tumours and they have a very high rate of recurrence after subtotal resection. Chordoid meningioma was also thought to be associated with many systemic features like Castleman’s disease and anaemia [5].
There are different mechanisms known contributing to the development of the extracranial meningiomas [6]. Arachnoidal cells which are present in the sheaths of nerves or vessels, during embryonal stages, emerging further through foramen, the displaced Pacchionian bodies get entrapped extracranially [7]. This displacement can occur following an event as trauma or cerebral hypertension which may further displace these arachnoid islets. the diverse pathological spectrum may be explained by their origin from multipotent cells. They may appear in soft tissues of face and neck, parotid gland, sinonasal tract, cranial bones, middle ear, scalp [8].
Symptoms like nasal obstruction, anosmia, facial pain, nasal discharge, epitaxis etc may be seen in meningiomas involving sinonasal tract [9]. The present case presented with nasal obstruction and episodes of epistaxis.
Chordoid meningiomas show trabeculae or cords of eosinophilic vacuolated cells in a myxoid matrix, which is also seen in the histological appearance of chordoma.
Chordoid meningiomas can present with diagnostic challenge if the histology or the imaging studies are not typical. Chordoma, chordoid glioma, myxoid chondrosarcoma (skeletal and extraskeletal), angiofibroma, myxopapillary ependymoma, and mucinous metastatic carcinomas enter in the differential diagnosis of this tumour [10]. Histopathology and immunohistochemistry are confirmatory.
Meningiomas are strongly immunopositive to vimentin, EMA and pancytokeratin. Mild positive reactions to CK7, S-100 protein, synaptophysin, CK20, GFAP, CD34, SMA, PCNA, progesterone receptor, and estrogen receptor may be seen [11].
The typical meningothelial cells in sheets as seen in our case help to differentiate histologically from chordomas which show classical multivacuolated physalipharous cells against an excessive mucoid background. Chordoma typically arises in the midline from the clivus and invariably associated with bone destruction. Brachyury appears to be a very sensitive and specific IHC marker for chordoma. Chondrosarcomas are generally negative for EMA and cytokeratin [12].
Myxopapillary ependymomas are specifically located in conus medullaris, cauda equina and filum terminale region and are GFAP positive. D2-40 marker has been used as one of the markers on IHC panel for the diagnosis of chordoid meningioma and was found to be positive in 75% of chordoid gliomas, 80% of chordoid meningiomas and in almost 100% of chondrosarcomas. [13].
Microcystic variant of meningioma shows certain degrees of myxoid stromal changes and cytoplasmic vacuolation. However, this variant lacks inflammatory cell infiltrates [14]. Thorough histopathological and immunohistochemical evaluation along with clinical and radiological findings are required for distinguishing chordoid meningioma from morphologic mimics.
Primary atypical meningioma involving nasal septum undergoing malignant transformation with distant metastasis into scalp and anterior chest wall has been reported in literature [15]. The aggressive nature of this tumour and its propensity for recurrence warrant an accurate diagnosis. Complete resection may not be possible on account of the location especially in large tumours. Postoperative radiotherapy has been advocated in such cases. In the present case, the tumour was completely excised and there was no recurrence on subsequent follow-up of 18 months.
Conclusion(s)
Chordoid meningioma is a rare and aggressive neoplasm. Recurrence rates are higher with incomplete excision of the tumour. Recognition of this uncommon variant is extremely important due to its unusual location and its potential for aggressive biological behaviour. A thorough histopathological and immunohistochemical workup in conjunction with clinical and radiologic findings is required to diagnose this entity so that optimal tumour resection can be achieved for better patient management.
Author Declaration:
Financial or Other Competing Interests: None
Was informed consent obtained from the subjects involved in the study? Yes
For any images presented appropriate consent has been obtained from the subjects. Yes
Plagiarism Checking Methods: [Jain H et al.]
Plagiarism X-checker: Oct 12, 2019
Manual Googling: Jul 13, 2020
iThenticate Software: Aug 27, 2020 (17%)
[1]. Kleihues P, Louis DN, Scheithauer BW, Rorke LB, Reifenberger G, Burger PC, The WHO Classification of Tumors of the Nervous SystemJournal of Neuropathology & Experimental Neurology 2002 61(2):215-25.10.1093/jnen/61.3.21511895036 [Google Scholar] [CrossRef] [PubMed]
[2]. Friedman CD, Costantino PD, Teitelbaum B, Berktold RE, Sisson GA, Primary extracranial meningiomas of the head and neckLaryngoscope 1990 100(1):41-48.10.1288/00005537-199001000-000102104554 [Google Scholar] [CrossRef] [PubMed]
[3]. Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, The 2007 WHO Classification of Tumours of the Central Nervous SystemActa Neuropathol 2007 114(2):97-109.10.1007/s00401-007-0243-417618441 [Google Scholar] [CrossRef] [PubMed]
[4]. Chang CY, Cheung SW, Jackler RK, Meningiomas presenting in the temporal bone: The pathways of spread from an intracranial site of originOtolaryngol. Head Neck Surg 1998 119(6):658-64.10.1016/S0194-5998(98)70030-0 [Google Scholar] [CrossRef]
[5]. Liu Y, Wang H, Shao H, Wang C, Primary extradural meningiomas in head: A report of 19 cases and review of literatureInt J Clin Exp Pathol 2015 8(5):5624-32. [Google Scholar]
[6]. Batsakis JG, Pathology consultation. Extracranial meningiomasAnn Otol Rhinol Laryngol 1984 93(3 Pt 1):282-83.10.1177/0003489484093003216732115 [Google Scholar] [CrossRef] [PubMed]
[7]. Lee DK, Kim DG, Choe G, Chi JG, Jung HW, Chordoid meningioma with polyclonal gammopathy. Case reportJ Neurosurg 2001 94(1):122-26.10.3171/jns.2001.94.1.012211147880 [Google Scholar] [CrossRef] [PubMed]
[8]. Swain RE, Kingdom TT, DelGaudio JM, Muller S, Grist WJ, Meningiomas of the paranasal sinusesAm J Rhinol 2001 15(1):27-30.10.2500/10506580178132941911258651 [Google Scholar] [CrossRef] [PubMed]
[9]. Dai J, Ma Y, Chu S, Le N, Cao J, Wang Y, Identification of key genes and pathways in meningioma by bioinformatics analysisOncol Lett 2001 15:8245-52. [Google Scholar]
[10]. Mori S, Oka K, Hakozaki H, Soga Y, Hayano M, Oka T, Chordoid meningioma. A case reportPathol Res Pract 2001 197(7):515-18.discussion 51910.1078/0344-0338-0012011482583 [Google Scholar] [CrossRef] [PubMed]
[11]. Kobata H, Kondo A, Iwasaki K, Kusaka H, Ito H, Sawada S, Chordoid meningioma in a child. Case reportJ Neurosurg 1998 88(2):319-23.10.3171/jns.1998.88.2.03199452243 [Google Scholar] [CrossRef] [PubMed]
[12]. Tirabosco R, Mangham DC, Rosenberg AE, Vujovic S, Bousdras K, Pizzolitto S, Brachyury expression in extra axial skeletal and soft tissue chordomas: A marker that distinguishes chordoma from mixed tumour/myoepithelioma/parachordoma in soft tissueAm J Surg Pathol 2008 32(4):572-80.10.1097/PAS.0b013e31815b693a18301055 [Google Scholar] [CrossRef] [PubMed]
[13]. Sangoi AR, Dulai MS, Beck AH, Brat DJ, Vogel H, Distinguishing chordoid meningiomas from their histologic mimics: An immunohistochemical evaluationAm J Surg Pathol 2009 33(5):669-81.10.1097/PAS.0b013e318194c56619194275 [Google Scholar] [CrossRef] [PubMed]
[14]. Dahmen HG, Studies on mucous substances in myxomatous meningiomasActa Neuropathol 1979 48(3):235-37.10.1007/BF00690527525263 [Google Scholar] [CrossRef] [PubMed]
[15]. Brat DJ, Scheithauer BW, Staugaitis SM, Cortez SC, Brecher K, Burger PC, Third ventricular chordoid glioma: A distinct clinicopathologic entityJ Neuropathol Exp Neurol 1998 57(3):283-90.10.1097/00005072-199803000-000099600220 [Google Scholar] [CrossRef] [PubMed]