AF is characterised by uncoordinated atrial activation with subsequent deterioration of atrial mechanical function. AF is the most common arrhythmia for which patients are hospitalised; approximately 33% of arrhythmia-related hospitalisations are for AF. Global prevalence is estimated to be 0.47%, but there is significant regional variation [1].
There is a greater prevalence in older persons and in heart failure, Coronary Artery Disease (CAD), VHD, obesity, diabetes, or Chronic Kidney Disease (CKD) [1,9-15]). AF genesis requires a vulnerable atrial substrate. Increased atrial pressure, volume overload and atrial structural remodeling, together with activation of the sympathetic and renin-angiotensin system causes AF in patients with structural heart disease. Some AF patients have no or minimal symptoms (25-40%), while many (15-30%) report severe or disabling symptoms [16,17].
Patterns of AF may vary from paroxysmal to persistent and permanent AF depending on the duration and mode of termination [Table/Fig-1]. The first documentation of AF is important as the treatment strategy depends on this. One must recognise that it may be asymptomatic and just a chance detection. It may be self-limited and do not tell about the duration of that particular episode, and neither does it preclude undetected episodes before the index episode. Only 10% of AF patients remained free of AF two years after incident AF, and recurrent (26%) or sustained AF (34%) were common [18].
In the Realise-AF survey which recruited 301 patients, it was found that the concomitant cardiovascular risk factors were high and the AF control was highly inadequate [19]. In the Nagpur pilot study, it was found that the AF prevalence was low compared to previous studies and that stroke prophylaxis was under utilised [20]. The Indian Heart Rhythm Society-AF (IHRS-AF) Registry of 1537 patients showed a significant mortality and morbidity in Indian patients due to AF [21].
Regional variation in healthcare systems, resource availability and physician preferences influence the therapies applied. Although the effect of AF on the quality of life and survival has been well documented in western population, similar data on incidence, prevalence, aetiology, mortality and morbidity in the Indian population is limited.
Hence, the primary aim of the present study is to estimate the incidence of MACE- ACS, cardiac death, stroke in patients with AF on one year follow-up.
Materials and Methods
This prospective cohort study included all patients above the age of 18 years with newly diagnosed AF between April 2016-March 2017 or previously documented (before April 2016) evidence of AF in ECG who presented or was admitted in the department of Cardiology, Trivandrum Medical College. Institutional Ethics Committee clearance was obtained (HEC.No.01/20/2016/MCT). Study procedures were in accordance with Helsinki Declaration. Informed written consent obtained from participants in their mother tongue.
Sample size was calculated using the below formula [22], p=8.8% (proportion of stroke among AF patients) with reference to the CHA2DS2VASc score Birmingham 2009 validation study [23].
p: Expected proportion (8.8%)
δ: Absolute precision (3%)
: Desired Confidence level=(95%)
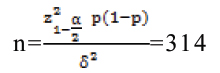
Transient AF due to reversible causes like during acute myocardial infarction, infection, metabolic abnormalities, alcohol intoxication, postoperative AF, critically ill patients with life expectancy less than seven days and those who did not give consent for enrolling into this study were excluded.
Study Method
Patients who presented with AF, either as the primary reason for their visit or as secondary diagnosis fulfilling the inclusion criteria were registered. Clinical data were collected by interviews with patients and review of medical records. At baseline, available data were collected based on the following points: patient demographics, medical history, type of AF (Valvular AF- moderate to severe Mitral stenosis, Mechanical Prosthetic Heart Valves and non-valvular AF- all others), date of diagnosis, symptoms, past events and treatment decisions. The diagnosis of all medical conditions including type of AF was based on the clinical records. Patients having AF was diagnosed by their ECG showing irregularly irregular QRS complex with no preceding P waves. Relevant blood investigations such as blood counts, renal parameters, liver function test, blood glucose, electrolytes, thyroid function tests, PT and INR, ECG and ECHO parameters were noted at baseline. Patients were followed-up at 1, 3, 6 and 12 months for symptoms, treatment adherence, investigations such as PT, INR, development of complications such as ACS, stroke, Transient Ischemic Attack (TIA), bleeding manifestations, and clinical course. For patients on oral anti-coagulation, the INR values at 1, 3, 6 and 12 months were collected. In patients with at least two available INR values, the degree of INR control was calculated and presented as the TTR by Traditional Method (i.e., Percentage of visits in range) [24]. It presents that how many visits had INR results in range, and divides by the total number of visits. Major bleeding is defined according to the International Society of Thrombosis and Haemostasis Bleeding Scale as fatal bleeding or symptomatic bleeding in a critical organ such as intracranial, intraocular, retroperitoneal, intraarticular or intramuscular with compartment syndrome or fall in Haemoglobin by ≥2 g/dL or requiring transfusion of ≥2 units of whole blood or red cells [25]. Data was collected from patients and bystanders using a semi-structured questionnaire-based interview [Annexure], clinical examination, lab investigations and telephonic follow-up. Investigations such as blood counts, renal parameters, liver function test, blood glucose, electrolytes, PT and INR, ECG and ECHO (Philips EPIQ 7) parameters were noted at baseline. Patients were followed-up with PT/INR values at follow-up visits, either by hospital visits or telephonic follow-up. The CHA2DS2VASc score [Table/Fig-2] was used to assess the risk of thromboembolism and to decide on anticoagulation in non-valvular AF.
Calculation of CHA2DS2VASc Score.
| Presence of risk factor | Score |
|---|
| Congestive heart failure | 1 point |
| Hypertension | 1 point |
| Age >75 years | 2 points |
| Diabetes mellitus | 1 point |
| Stroke/TIA | 2 points |
| Vascular disease | 1 point |
| Age 65-74 years | 1 point |
| Sex (female) | 1 point |
TIA: Transient Ischemic Attack
Presence of each of the risk factor attributes to the above given points and the total points added up to get the CHA2DS2VASc score which predicts the risk of thromboembolism in Non-valvular-AF patients and aids in deciding anticoagulation therapy.
Statistical Analysis
All data are expressed as mean and standard deviation. Data were summarised in subgroups using measures of central tendency and dispersion, number of patients for continuous data and as count and percentage for categorical data. Data analysis was done with the help of Excel 2010 statistical software.
Results
There were total of 346 patients with AF in this study. Average age of the patients was 60.5 years (SD of 11.5 years) with age ranging from 22 years to 87 years [Table/Fig-3]. Females outnumbered males and constituted 59.5% of the study population. Overall, hypertension was the most prevalent comorbid condition (44.5%). History of VHD was present in 38.2% (n=132) of the patients, out of which 27.2% (n=94) were due to RHD. Dilated Cardiomyopathy, Hypertrophic and Restrictive cardiomyopathy were present in 5.2%, 1.7% and 0.6% of the study population, respectively. CAD was present in 20.2% of which 20% (n=14) of CAD patients had undergone Percutaneous Coronary Intervention (PCI). Cardiac surgery which included Mitral Valvotomy, Valve Replacement and Coronary Artery Bypass Graft (CABG) constituted 9.2% (n=32) and congenital heart disease patients formed only 1.7% (n=6) of the study population [Table/Fig-4]. Electrocardiogram findings include significant Q wave in 6.9% of the patients, Left Bundle Branch Block (LBBB) in 4%, Left Ventricular Hypertrophy (LVH) in 19.7% and 23.7% had significant ST-T changes.
Age distribution of AF patients.
| Age in years | Frequency | Percentage |
|---|
| <50 | 54 | 15.6 |
| 50-74 | 258 | 74.6 |
| >75 | 34 | 9.8 |
| Total | 346 | 100 |
Baseline characteristics.
| Baseline characteristics | Value (n) | Percentage |
|---|
| Male | 140 | 40.5% |
| Female | 206 | 59.5% |
| Hypertension | 154 | 44.5% |
| RHD | 94 | 27.2% |
| CAD | 70 | 20.2% |
| COPD/Asthma | 48 | 13.9% |
| Hypothyroid | 26 | 7.5% |
| Hyperthyroid | 10 | 2.9% |
| Diabetes | 68 | 19.7% |
| Cardiomyopathy (Total) | 26 | 7.5% |
| -Dilated cardiomyopathy | 18 | 5.2% |
| -Hypertrophic cardiomyopathy | 6 | 1.7% |
| -Restrictive cardiomyopathy | 2 | 0.6% |
| Previous cardiac surgery | 32 | 9.2% |
| Congenital heart disease | 6 | 1.7% |
| Chronic kidney diseases | 16 | 4.6% |
RHD: Rheumatic heart disease; CAD:Coronary artery disease; COPD: Chronic obstructive pulmonary disease
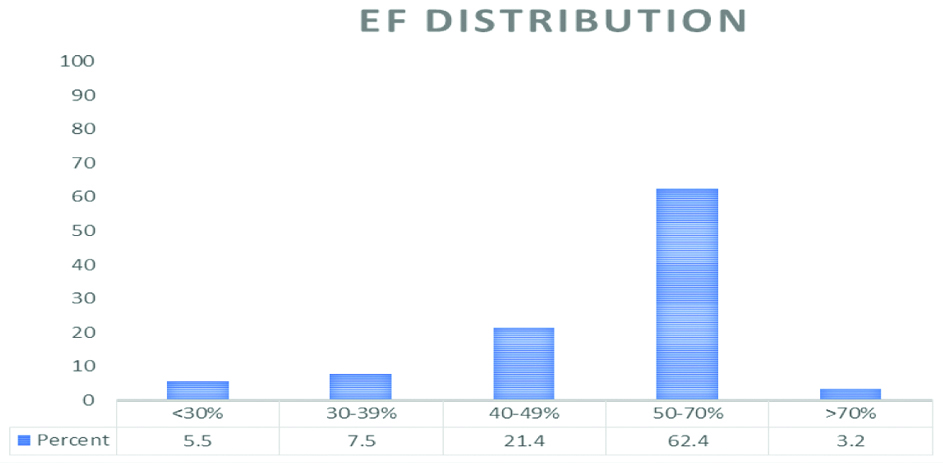
Echocardiogram findings include Left Atrial (LA) dilatation (LA diameter ≥4 cm) in 65.03% of the patients. Regional wall motion abnormality was present in 12.7% (44 patients) and Left Ventricular dysfunction (LV ejection fraction <50%) was present in 34.4% of patients [Table/Fig-5]. Mitral stenosis (Mitral valve area <1.5 cm2) was present in 23.1% (n=80); all were due to RHD. Aortic stenosis was present in 4% (n=14). Mitral Regurgitation in 29.5% (n=102) and Aortic Regurgitation in 8.7% (n=30). LV Hypertrophy was present in 29.2% (n=101) and Pulmonary Hypertension in 25.4% (n=88).
Distribution of LV systolic function.
EF: Ejection fraction; Y axis: number of Patients; X-axis: EF distribution
In total, 74% (n=256) received oral anticoagulants (vitamin K antagonists). All patients were on vitamin K antagonists and none were on Novel Oral Anticoagulants (NOACs). The CHA2DS2VASc score was used in 67.9% to assess the risk of thromboembolism and to decide on anticoagulation in non-valvular AF. Aspirin was given to 34.7% of the patients (n=120) and 9% of the patients were on dual anti platelet therapy. Beta blockers (n=290) and Digoxin (n=88) were the most commonly used drugs for rate control. Angiotensin Converting Enzyme (ACE) inhibitors (26.6%), Angiotensin II Receptor Blockers (ARBs) (13.9%) and diuretics (43.9%) were the other commonly used drugs. Total of 12 patients had Permanent Pacemaker Implanted (PPI), the primary indication being Sick Sinus Syndrome (SSS).
Time in Therapeutic Range (TTR) with INR values between 2 to 3 was calculated by Traditional method. Only a single INR value was available in 23 patients. TTR ≥50% was present in 40.9% patients. TTR >60% (good control) was present in only 9.2% of the study population. Intermediate control (TTR 50-60%) was noted in 31.7% and poor control (<50%) in 59.1% of the patients [Table/Fig-6]. Mean TTR was 28.12%.
Time in Therapeutic Range (TTR).
| TTR | Frequency (total=186) | Percent |
|---|
| 0% | 49 | 26.3% |
| 25% | 61 | 32.8% |
| 50% | 59 | 31.7% |
| 75% | 15 | 8.1% |
| 100% | 2 | 1.1% |
| Total | 186 | 100% |
During the follow-up period of one year, 17 patients were lost to follow-up. Of the study population, 14.6% had AF related death [Table/Fig-7]. Embolic stroke developed in 9.4% of the patients and ACS occurred in 2.7%. None of the patients had peripheral embolisation. MACE occurred in total of 26.7% of AF patients. Major bleeding occurred in 8.8% of the patients. Overall, mortality during the study period was 15.8% of which four deaths were unrelated to AF.
MACE and major complications on follow-up (n=329).
| Events | 1 month (n) | 3 months (n) | 6 months (n) | 12 months (n) (%) |
|---|
| Overall MACE | 9 | 28 | 47 | 88 (26.7) |
| ACS | 0 | 2 | 5 | 9 (2.7%) |
| Cardiac death | 5 | 11 | 19 | 48 (14.6%) |
| Stroke (Embolic) /TIA | 4 | 15 | 23 | 31 (9.4%) |
| Death | 5 | 12 | 21 | 52 (15.8%) |
| Major bleeding | 2 | 6 | 17 | 29 (8.8%) |
| Peripheral embolism | 0 | 0 | 0 | 0 |
| Intracranial haemorrhage | 0 | 1 | 1 | 2 (0.6%) |
MACE: Major adverse Cardiovascular events; ACS: Acute Coronary Syndrome; TIA: Transient Ischemic Attack
Discussion
The incidence of AF increases with advancing age. In this study, more than two third of the patients were above 50 years. Mean age was 60.5±11.5 years. AF is the most common sustained arrhythmia present in 3-5% of those >65 years of age [4,26]. Average age in the RELY-AF Registry was 65.9±14.8 years [14] and in the recent IHRS AF registry [21] which enrolled 1537 AF patients mean age of the entire Indian cohort was 54.7±15.9 years. In this study, the proportion of females (59.5%) were more, as in the IHRS-AF registry (51.5%) [21].
Hypertension is the most common risk factor for AF, globally. In this study, hypertension was present in 44.5%. Hypertension was identified in 41.6% of Indian AF patients in the RELY-AF Registry [14] and in 31.4% in the IHRS-AF registry [21]. Hypertension is most likely to be untreated and poorly controlled and in this study, 19.7% patients had evidence of LV hypertrophy; a marker of prolonged uncontrolled hypertension and poor outcomes. This leads to elevated LV end diastolic pressures, diastolic dysfunction and elevated LA pressures leading to Atrial dilatation and AF. Thus, effective strategies to detect, treat and control hypertension need to be implemented to prevent AF.
RHD remains an important cause of AF in India. In this study, 26.6% of patients had ECHO evidence of moderate to severe VHD of Rheumatic aetiology of which majority were contributed by Mitral Stenosis. Thus, VHD attributed to one third of the cases, while it was the commonest cause (47.6%) in IHRS-AF Registry [21]. In RELY-AF Registry, it was present in 31.5% of AF patients in India [14]. Mitral stenosis patients with AF are at a higher New York Heart Association (NYHA) class of dyspnoea and show greater LA enlargement. The other attributing factors for thrombus formation in AF are Heart Failure present in 20.8%, diabetes in 19.7% and CAD in 20.2% in the present study. In IHRS-AF study, these figures were 18.7%, 16.1% and 16.2%, respectively [21].
In patients with enlarged and dysfunctional atria, despite correction of the underlying valvular lesion, AF often persists. In this study, 9.2% of patients had undergone previous cardiac surgery. Strategies for early diagnosis and preventing the development and recurrence of RHD have a great impact on preventing AF and its complications [27].
Paroxysmal episodes of non-valvular AF remain as paroxysmal for long periods and rarely progress to persistent. However, though the valvular AF begins as paroxysmal episodes, they rapidly progress to persistent AF. There was a substantial variation in the type of AF in various studies. In RELY-AF Registry paroxysmal AF was present in 20.5%, persistent in 32.9% and permanent in 46.6% [14]. While in the IHRS AF Registry paroxysmal, persistent and permanent were 20.4%, 33.0% and 35.1%, respectively [21]. In present study group it was 34.7%, 23.1% and 42.2%, respectively. In this study, Valvular AF (Moderate to Severe Mitral Stenosis and Mechanical Prosthetic Valve) constituted 26.6% and Non-valvular AF in 73.4%.
Occurrence of AF correlates with LA size; incidence rises from 3% when LA diameter is <40 mm to 54% when LA diameter is >40 mm [28]. LA enlargement was present in 65.03% of AF patients in this study. Chronic AF per say can cause Atrial enlargement.
AF is a major cause of systemic thromboembolism. In patients over the age of 65 years, it is responsible for more than one third of all strokes [29]. Advancing age, previous thromboembolic events, presence of VHD, heart failure, enlarged LA, previous MI, hypertension and LA thrombus on echocardiography predict occurrence of embolic stroke in patients with AF [30]. Presence of AF multiplies the risk of stroke five times in a patient with structurally normal heart, and increases by a factor of 17 in those with VHD. Risk of recurrent strokes appears to be similar with chronic and paroxysmal AF.
The appropriate use of oral anticoagulation can reduce the rate of stroke by two third. Current ESC guidelines recommend that in Non-valvular AF, if the patient has a CHA2DS2-VASc score of ≥2, anticoagulants are indicated to reduce thromboembolic events [23]. Vast majority of the non-valvular AF patients had a high CHA2DS2VASc score ≥2 (65.1%) almost similar (68.5%) to that in IHRS-AF registry [21]. However, OAC were prescribed for only 58% of these patients with non-valvular AF and a CHA2DS2VASc score of ≥2 in the RELY AF Registry [14]. In this study, OACs were not used in 32.9% of non-valvular AF with CHA2DS2VASc score ≥2, and the rest 67.1% received OAC. In patients with VHD all were on OAC. In total 74% of all AF patients were on OAC. There was also a tendency to under anticoagulate. Good control (TTR >60%) was present in only 9.2%. Use of OACs and proper dosing in these AF patients at risk for stroke are limited by infrequent access to INR testing, irregular follow-up visits, increased risk of bleeding and non-compliance to medication.
In addition to lowering of stroke risk, there is evidence that Warfarin leads to less severe stroke episodes and lowers the 30-day stroke mortality [31]. In RELY study, INR control was particularly poor in India, China and Southeast Asia with mean TTR of 33.7% [14]. Present study has a mean TTR of 28.12%. NOACs help to overcome some of these barriers; however, high costs make them affordable for only few patients. Although aspirin is less effective than oral anticoagulants, it does not require regular monitoring and is affordable, thereby though not ideal but is still better than no stroke prevention therapy at all.
Important safety issue with the use of anticoagulants is the increased risk of major bleeding leading to hospitalisation, transfusion, surgery, or involving crucial anatomic locations. Major bleed occurred in 8.8% of patients during one year follow-up. Intracranial Haemorrhage (ICH) is the most serious bleeding complication accounting for high morbidity and mortality. ICH in anticoagulated population occurs at a rate of 0.2% to 1.0% per year [32]. This study had two patients (0.6%) who developed ICH.
The management of AF is aimed at either control of ventricular rate without attempting to restore sinus rhythm, or restoration to sinus rhythm with follow-up aggressive therapy to maintain it. Rhythm-control strategy offers no survival advantage over the rate-control strategy (which has lower risk of adverse drug effects). In both approaches, the use of anticoagulant drugs is recommended [33]. In this study, rate control strategy was adopted in 93.6% and only 5.8% had rhythm control.
In this study, at one year follow-up, MACE occurred in 26.7%, which is higher than that in a previous study conducted in China (one-year MACE was 21.9%) [34]. Stroke occurred in 9.4% while 1.03% patients had stroke in the IHRS-AF Registry [21]. Of these patients who developed stroke in this study, 90.32% were on OAC and 6 patients had CHA2DS2VASc score <2. Subtherapeutic INR and drug default were the contributors for this high rate of stroke. A study by Healey JS et al., showed one-year mortality rate of around 11-20%, with heart failure being the most common cause of death [35]. In this study, AF related death occurred in 14.6% with heart failure being the commonest cause for the death. While in the IHRS-AF registry, the mortality at one year was 6.5% with heart failure being the commonest cause [21]. ACS accounted to 14% of the mortality in IHRS-AF registry [21]. In this study, ACS occurred in 2.7% of the AF patients and attributed to 3.8% of the total mortality.
Limitation(s)
The possibility of selection bias cannot be excluded as each consecutive patients presenting to the cardiology department could not be recruited. This study represents patients from a tertiary care centre, managed by cardiologists hence confounds the community incidence and prevalence of AF.
Conclusion(s)
In contrast to the previous studies non-valvular AF was thrice as common as valvular AF. Though three fourth of the patients were on oral anticoagulants, <10% had their INR under good control. The follow-up data shows high mortality and event rates in this cohort of patients due to subtherapeutic anticoagulation. This study underlines the importance of early detection and treatment of underlying risk factors of AF. Patients on OAC, particularly VKAs have to do frequent monitoring of INR to ensure adequate anticoagulation. The advent of NOACs with a superior anticoagulation profile is definitely a boon for AF patients particularly in view of the need for prolonged duration of therapy.
RHD: Rheumatic heart disease; CAD:Coronary artery disease; COPD: Chronic obstructive pulmonary disease
MACE: Major adverse Cardiovascular events; ACS: Acute Coronary Syndrome; TIA: Transient Ischemic Attack