Soft tissue vascular anomalies are predominant paediatric diseases, representing some of the most difficult diagnostic challenges in the field of diagnostic and intervention radiology with a reported worldwide incidence of roughly 0.3% [1]. The role of imaging is indispensable, and its primary goals include lesion characterisation and evaluation of perfusion characteristics and anatomic extent of the disease, thus aiding in therapeutic decision-making. Treatment options may depend on the site, size, and complexity of the lesion, and improper intervention may lead to recurrence or aggravation of the lesion [2].
The US is the preferred imaging modality for initial assessment and characterisation of such soft tissue lesions of presumed vascular origin, as it is safe, non invasive, rapid, and relatively inexpensive. Colour and spectral Doppler evaluations are useful in the assessment of vascular flow characteristics and velocities [3].
The MRI is also highly valuable in the characterisation of vascular anomalies and is particularly useful in the evaluation of the extent and anatomic relationship of these lesions to adjacent structures. The use of contrast-enhanced MR angiography with 3D T1-weighted fast gradient-echo sequence effectively evaluates lesion perfusion, thus aiding further in characterising lesions. However, MRI is relatively expensive, time consuming and requires contrast administration for functional analysis and better lesion characterisation [4].
The optimal imaging modality when characterising and screening lesions especially in paediatric patients remains a contentious issue due to these distinct advantages and limitations. Thus, this study aimed to characterise soft tissue vascular anomalies based on the imaging features of two modalities-US and CEMRI. The intra-observer agreement of these diagnostic modalities with histopathology was also compared with respect to the detection of lesions which may help in selection of appropriate imaging modality when characterising and screening such lesions.
Materials and Methods
This study was a prospective single institutional study undertaken in a tertiary care hospital for over a period of two years from August 2012 to July 2014. Institutional Ethical Committee approval (108/2012/57) was acquired, and all eligible patients and guardians received a full explanation of the nature and purpose of the study. Written informed consent was obtained from all subjects. In paediatric patients, the consent was acquired from parents or guardians.
Inclusion criteria: We enrolled 75 subjects with soft tissue vascular anomalies. Subjects of all ages were included in the study. The following two categories of vascular anomalies were included: vascular tumours (with haemangioma as the most common) and vascular malformations which were largely subcategorised based on their flow dynamics as venous, lymphatic, and Arteriovenous Malformations (AVMs). Among these patients, 71 underwent both US and MRI, three underwent US alone, and one patient underwent MRI alone.
Exclusion criteria: Patients with intracranial and spinal vascular anomalies were excluded from the study. A detailed clinical history was taken and additional details including lesion location, size, multifocality, colour, presence of bruit, ulceration, prominent veins, and any presence of deformity were documented.
Ultrasound (US) Technique
The US studies were performed without sedation on ACUSON Antares Colour Doppler US System (Siemens Healthineers, Germany) using linear transducer with imaging and Doppler frequency of 7 MHz. Greyscale evaluation was done followed by colour and spectral Doppler imaging. Following characteristics were evaluated in all lesions: size, calcification, arterial and venous flow, peak velocity, and vessel density (number of vessels/cm2).
MRI Technique
The MRI was performed on a 1.5-T scanner (MAGNETOM AVANTO, Siemens Healthineers) using dedicated coils. A comprehensive routine imaging protocol was based on isotropic T2 and T1-VIBE sequences (both with and without fat suppression), as shown in [Table/Fig-1]. For contrast-enhanced images, time-resolved high-spatial-resolution multiphase three-dimensional MR angiography with subsequent image subtractions was performed. Dynamic CEMRI were synchronised with intravenous injection of Gadobenate dimeglumine at a dose of 0.2 mL/kg of body weight at an injection rate of 2 mL/s using a pressure injector. In infants, intravenous contrast bolus was injected manually by hand slowly followed by saline flush. Dynamic contrast-enhanced images were obtained continuously every 5 seconds, for a total of 120 seconds. In the MR image interpretation, the following features were elucidated: lesion conspicuity, border, margin, appearance of nidus, presence of flow voids, presence of adjacent soft tissue and neurovascular bundle involvement, feeding and draining vessels, artery-lesion enhancement time, contrast rise time, and any other abnormality in the area scanned.
Sequence parameters of MRI protocol used to assess peripheral vascular malformations.
| Imaging sequence | TR/TE (ms) | Flip angle (°) | Slice thickness (mm) | Matrix (pixel) | FOV (mm) | NEX |
|---|
| Fat-suppressed T1- weighted fast spin-echo | 420-820/14-20 | 30 | 4-8 | 256×256 | 180-380 | 1 |
| Fat-suppressed T2- weighted fast spin-echo | 3800/96-120 | 30 | 4-8 | 256×256 | 180-380 | 1-2 |
| T1-weighted 3D gradient echo | 4.6-6.8/1.8-3.6 | 25-30 | 3.8-5.2 | 115-175×160-256 | 180-380 | 1 |
*FOV: Field of view; NEX: Number of excitations
All patients underwent a subsequent histopathological analysis within three months of examination. Digital subtraction angiography correlation was done when interventional therapy was contemplated or when there was diagnostic ambiguity in few cases.
Statistical Analysis
Data analysis included evaluation of patients’ demographic profile. Quantitative variables between the study groups were compared using an unpaired t-test. Weighted kappa was computed to assess the agreement between US and MRI versus histopathology. ROC curves were obtained by calculating the cut-off point of the peak arterial and venous flow velocity for differentiating AVMs from other vascular anomalies. The sensitivity, specificity, and accuracy were calculated from the ROC curves. The p-value <0.05 was considered statistically significant. SPSS version 20.0 was used for all analyses.
Results
In this study, 75 patients with soft tissue vascular anomalies i.e., 39 males (52%) and 36 females (48%) were included. The mean age of the study subjects was 19.12±15.02 years, ranging from 2 months to 50 years. The predominant study subjects were of paediatric age group i.e., 25 patients in the age group 0-10 years, followed by 15 patients each in age groups 10-20 and 20-30 years, 9 patients in age group 30-40 years and 11 patients in age group 40-50 years. No significant difference was noted in the distribution of vascular anomalies between sexes. Local swelling was the most common presenting clinical feature (n=72; 96%), followed by pain (n=33; 44%) and cosmetic deformity (n=24; 32%). Functional impairment was found in 22 patients (29%). Phlebectasia (n=9, 12%) and bony involvement (n=9; 12%) was also noted.
In this study, vascular anomalies evaluated were vascular tumours (with infantile haemangioma as the most common) and vascular malformations which were subcategorised as venous, lymphatic, and AVMs. Other tumours, which comprised of two haemangioendotheliomas and one tufted angioma, were categorised into other vascular tumours. Vascular malformations were also subcharacterised based on presence of arterial component into high and low-flow malformations. Venous malformations accounted for the highest number of cases, i.e., 26 (34.66%) [Table/Fig-2].
Number of cases and percentages of different types of vascular anomalies.
| Types of vascular anomalies | Number (n) | Percentage (%) |
|---|
| Venous malformations | 26 | 34.66 |
| Arterio-Venous Malformations (AVM) | 20 | 26.7 |
| Infantile haemangioma | 17 | 22.7 |
| Lymphatic malformations | 9 | 12 |
| Other vascular tumours | 3 | 4 |
| Total | 75 | 100 |
The lesions were evaluated by greyscale imaging (soft tissue, cysts, and vascular channels). Presence of soft tissue in vascular malformations was found in all 17 cases of haemangiomas, three cases of other vascular tumours, and two cases of venous malformations. Conversely, none of the AVMs showed soft tissue component. Cystic spaces were observed only among the nine cases of lymphatic malformations. Vascular channels on greyscale evaluation were seen in all the 20 cases of AVMs, 25 cases of venous malformations, and four cases of haemangiomas.
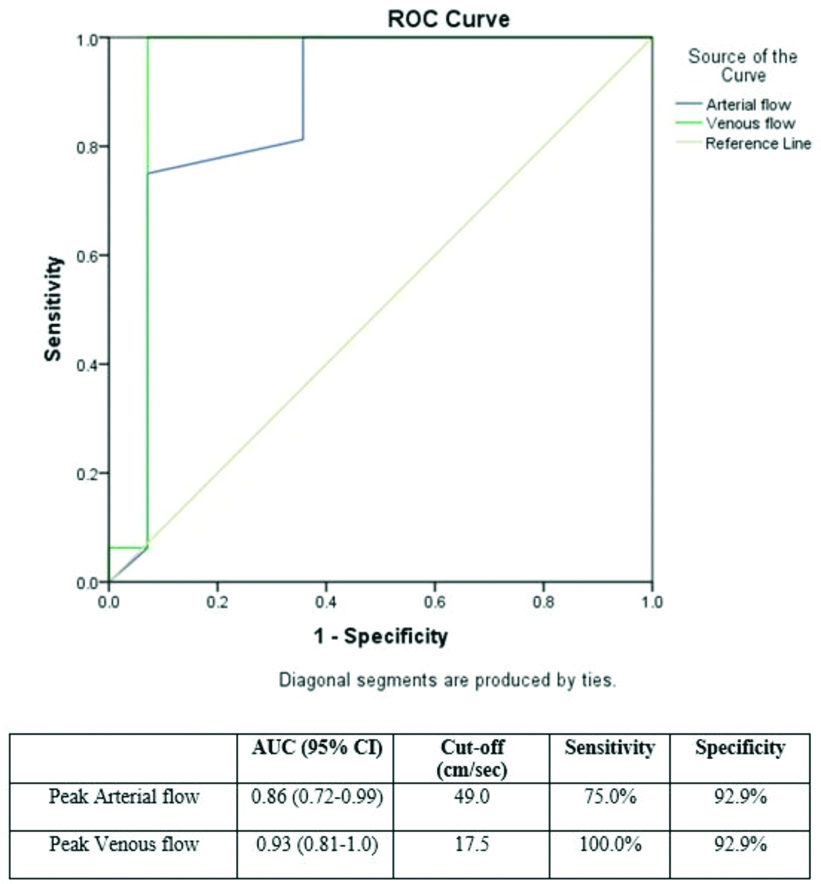
Doppler sonographic detectable arterial flow was noted in all AVMs and 90% of haemangiomas, but was absent in venous and lymphatic malformations. Peak arterial and venous flow velocities were calculated. The mean peak arterial flow rates in AVMs, venous malformations, and haemangiomas were 55.63, 44, and 42.46 cm/s, respectively. The mean peak venous flow rates in AVM, venous malformations, and haemangiomas were 30, 5.26, and 10.26 cm/s, respectively. Using ROC curve to differentiate AVM from other vascular anomalies, using peak arterial flow, the Area Under the Curve (AUC) was 0.86 (95% Confidence Interval (CI) 0.72-0.99). At a cut-off value of 49.0 cm/s, the sensitivity was 75% and specificity was 92.9%. For peak venous velocity, the AUC was 0.93 (95% CI 0.81-1.0). At a cut-off value of 17.5 cm/s, the sensitivity was 100.0% and the specificity was 92.9% [Table/Fig-3]. Moreover, 90% of the AVMs revealed high vessel density and the remaining cases showed medium vessel density. Vessel density was evaluated using a method described by Paltiel HJ et al., i.e., low (<2 cm2), moderate (2-4 cm2), and high (≥5 cm2) [3]. Furthermore, in 18 AVMs (90%), high vessel density was observed. In two cases, medium vessel density was noted. None of the AVMs showed low vessel density. In addition, 56% of venous malformation lesions revealed low vessel density. In haemangiomas, eight cases showed high, four showed medium, and rest showed low vessel density. Phleboliths were noted in both venous malformations and haemangiomas. However, none of the AVMs showed phleboliths.
ROC curve for peak arterial and venous flow to differentiate AVM from haemangiomas.
MR Findings
The MRI was performed in 72 patients, and imaging characteristics on conventional sequences were evaluated for the presence of soft tissue, flow voids, nidus with plexiform connection, fluid-fluid level, phlebolith, cystic spaces, lacy tangles, and tense tangles. Findings are summarised in [Table/Fig-4].
Conventional MR characteristics among different vascular anomalies.
| Type of vascular anomalies | Conventional MR Characteristics (T1WI, T2WI, STIR, GRE) (n) |
|---|
| Soft tissue | Flow voids | Nidus with plexiform connection (N-PX) | Fluid-fluid level | Phlebolith | Cystic spaces | Lacy tangles | Tense tangles |
|---|
| Venous malformations | 5 | 1 | 0 | 12 | 20 | 0 | 23 | 0 |
| Arterio-Venous malformations | 0 | 20 | 20 | 0 | 0 | 0 | 0 | 17 |
| Haemangioma | 17 | 15 | 0 | 2 | 3 | 0 | 2 | 0 |
| Lymphatic malformations | 0 | 0 | 0 | 5 | 1 | 9 | 0 | 0 |
| Other vascular tumours | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Total | 22 | 36 | 20 | 19 | 24 | 9 | 25 | 17 |
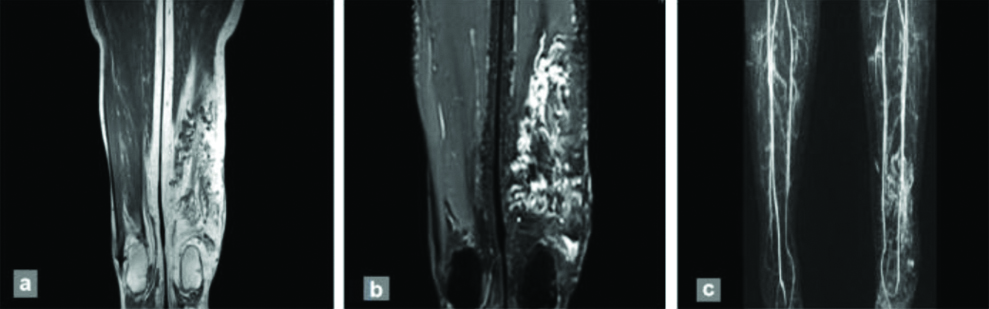
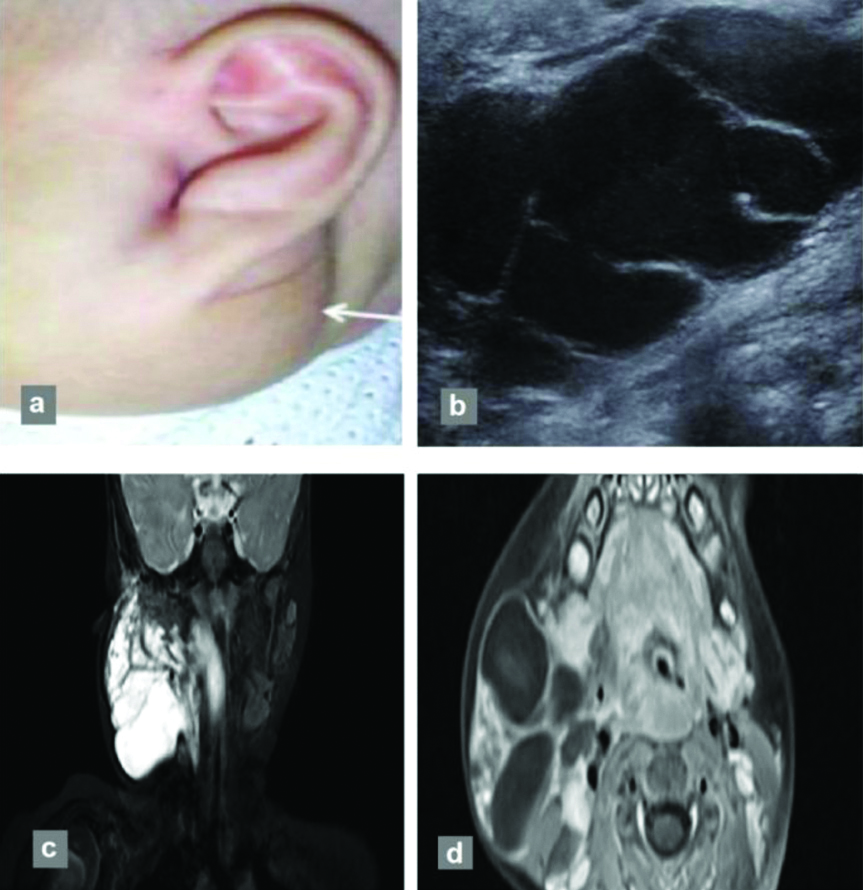
Further, the phase of maximum enhancement was evaluated to differentiate the anomalies, including arterial, early venous, intermediate venous, delayed venous and delayed phases acquired on post contrast MRI. All AVMs and two haemangiomas exhibited maximum enhancement in the arterial phase. In addition, 12 of the haemangiomas showed maximum enhancement in intermediate venous phase, while all venous malformations were maximally enhanced in the delayed venous phase [Table/Fig-5]. A magnetic resonance image of a 24-year-old female with swelling in posterior aspect of left lower leg (venous malformation) with delayed enhancement is shown in [Table/Fig-6]. Lymphatic malformations showed peripheral enhancement of the cystic component, which was most evident in the delayed phase. The cystic component and peripheral enhancement in a lymphatic malformation in an eight-month-old female is shown in [Table/Fig-7].
Phase of maximum enhancement in vascular anomalies.
| Type of vascular anomalies | Phase of maximum enhancement (n) |
|---|
| Arterial | Early venous | Intermediate venous | Delayed venous | Delayed |
|---|
| Venous malformations | 0 | 0 | 0 | 26 | 0 |
| Arterio-Venous malformations | 20 | 0 | 0 | 0 | 0 |
| Haemangiomas | 2 | 3 | 12 | 0 | 0 |
| Lymphatic malformations | 0 | 0 | 0 | 2 | 7 |
| Other vascular tumours | 3 | 0 | 0 | 0 | 0 |
| Total | 25 | 3 | 12 | 28 | 7 |
A 24-year-old female with venous malformation presenting as swelling in posterior aspect of left lower leg: (a) Coronal T2WI showing serpiginous hypointense flow voids representing vascular channels; (b) and (c) Postcontrast T1FS and 3D contrast angiography showing enhancement on delayed phase.
An eight-month-old female with lymphatic malformation, presenting with neck swelling: (a) from birth (white arrow) without any thrill on palpation; (b) Greyscale US image showing cystic lesion with internal septations; (c) Multiloculated cystic lesion showing fluid-fluid level and delayed peripheral enhancement on coronal T2FSWI; and (d) axial postcontrast T1WI showing peripheral enhancement.
Vascular flow voids were observed in all AVMs and 15 haemangiomas. Thus, the association was statistically insignificant (p=0.459) in differentiating between AVM and haemangiomas. Fluid-fluid levels were present in both venous and lymphatic malformations and cannot be used to differentiate the two conditions (p=1.000). Contrast washout was found in all AVMs, but not in haemangiomas and low-flow vascular malformations. Feeding vessels were identified in all AVMs and in 14 haemangiomas. In AVMs, the feeding vessel patterns were disorganised and tortuous, but those in haemangiomas were organised and non tortuous. Early draining veins were found in all AVMs and in six haemangiomas, but were completely absent in venous and lymphatic malformations. Arterial-lesion enhancement time was ≤5 s in all AVMs and in 53% of haemangiomas. Thus, this finding was not statistically significant in differentiating the two conditions (p=0.324).
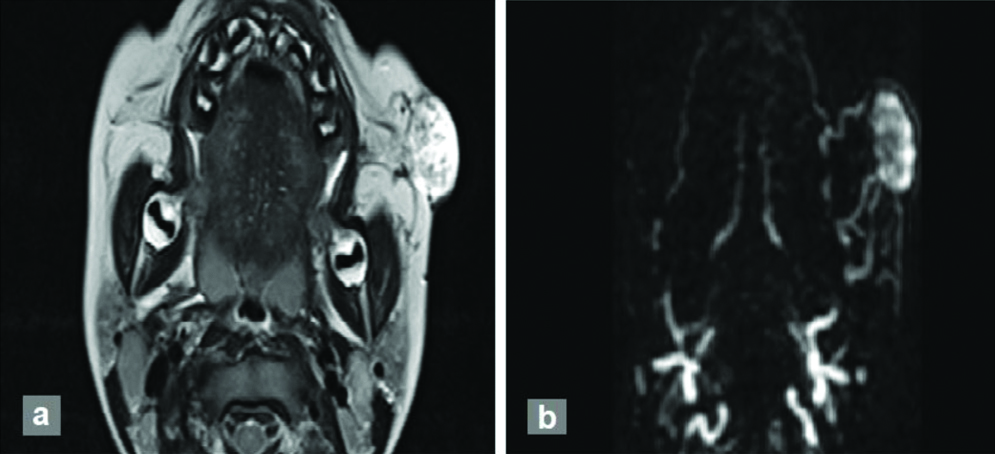
However, none of the venous malformations revealed arterial-lesion enhancement time of ≤5 s. Increase in contrast rise time was evaluated in all cases. In this study, 100% of AVMs showed contrast rise time <20 s. None of the other malformations showed such an early contrast rise time [Table/Fig-8]. A AVM in a three-year-old male with swelling in left cheek showing early arterial enhancement is shown in [Table/Fig-9].
Different ranges of contrast rise time in different vascular anomalies.
| Type of vascular malformations (n) |
|---|
| Contrast rise time (seconds) | Venous malformations | Arterio-Venous malformations | Haemangioma |
|---|
| 0-<10 | 00 | 15 | 00 |
| ≥10-<20 | 00 | 05 | 00 |
| ≥20-<30 | 00 | 00 | 00 |
| ≥30-<40 | 01 | 00 | 08 |
| ≥40-<50 | 00 | 00 | 05 |
| ≥50-<60 | 00 | 00 | 03 |
| ≥60-<70 | 00 | 00 | 00 |
| ≥70-<80 | 10 | 00 | 01 |
| ≥80-<90 | 11 | 00 | 00 |
| ≥90-<100 | 04 | 00 | 00 |
| Total | 26 | 20 | 17 |
Three-year-old male with AVM presenting as swelling in left cheek: (a) T2 hyperintense lesion with tortuous vascular flow voids; (b) 3D contrast angiography shows early arterial enhancement with feeding vessels arising from left facial artery and venous drainage into jugular venous system.
Comparison of US and CEMRI
A final diagnosis was given for each US and MRI, and their level of similarity was compared with the final histopathological diagnosis. The weighted kappa values were calculated. Both US and MRI had very high (almost perfect) agreement with histopathology with the following values: kappa=0.884, SE=0.045, 95% CI (0.794-0.973) for US, and kappa=0.923, SE=0.037, 95% CI (0.850-0.996) for MRI.
Discussion
The US and MRI are both valuable imaging modalities widely utilised in the assessment and classification of soft tissue vascular anomalies [5]. A multidisciplinary approach inclusive of clinical evaluation, imaging appearance, and evaluation of flow characteristics assumes paramount importance for therapeutic planning and clinical prognosis [6]. In the present study, a slight male preponderance was observed with a male/female ratio of 1.08. Venous malformations were the most common vascular anomaly. However, few previous studies reported that the prevalence of haemangiomas was higher than that of venous malformations [7-9]. This could be because most of the paediatric haemangiomas are readily diagnosed clinically and thus were not referred for imaging studies, leading to an inclusion bias. The AVMs, lymphatic malformations, haemangioendotheliomas, and tufted angiomas were other lesions encountered in this study. Vascular anomalies were found to be more common in the head and neck region, followed by those in the extremities. In addition, 85% of AVMs were located in the head and neck region. Most previous studies show a similar distribution pattern [3,10].
Intrathoracic and intra-abdominal extension was found in venous and lymphatic malformations, while osseous involvement was noted in venous malformations and haemangiomas in this study. Multifocality was noted in venous malformations and haemangiomas. Samlaska CP and Gagliardi JA, and Justin QL et al., have mentioned about the osseous involvement in venous malformation and haemangioma similar to the present study [11,12].
The difference in therapeutic approach and imaging overlap in AVMs and haemangiomas makes accuracy in classification essential. On greyscale US, the presence of the soft tissue component helped distinguish haemangioma from AVM. This was in concordance with studies by Bittles MA et al., and Leng T et al., [13,14]. The soft tissue component was also noted in two cases of venous malformations.
In this study, the mean peak arterial and venous flow rates in AVMs were higher than those in haemangiomas. A cut-off venous peak velocity of 17.5 cm/s showed 100% sensitivity in the detection of AVM over other anomalies. This could be of high value in differentiating AVM from haemangioma, especially when the lesion is atypical and other features are equivocal.
On the other hand, maximal AVM showed high vessel density; however, it was statistically not significant in differentiating venous malformations from haemangiomas. Phleboliths were noted in venous malformations and haemangiomas, but were absent in AVM, in concordance with the finding of Paltiel HJ et al., [3]. Doppler-detectable arterial flow was noted in AVM and haemangioma, but was absent in low-flow vascular malformation. This was similar to findings of previous studies [3,15].
On conventional MR, flow voids were found in all AVMs and in 15 haemangiomas, suggesting that the association was not statistically significant; however, high-flow and low-flow vascular anomalies could be differentiated using this criterion, in accordance with results of Donnelly LF et al., and van Rijswijk CS et al., [16,17].
Fluid-fluid levels were present in both venous and lymphatic malformations; moreover, cystic component was noted in all cases of lymphatic malformations, similar to previous studies [5,10].
The AVMs were observed as highly enhancing lesions in the present study with disorganised and tortuous pattern of feeding vessels showing early washout of contrast. On contrary, persistent enhancement was noted in haemangiomas as well as venous malformations. Similar pattern of washout was noted in previous comparative studies by Bittles MA et al., Leng T et al., and Tao Q et al [13,14,18].
Early draining veins were found in all AVMs and in some haemangioma cases, but were not found in venous and lymphatic malformations. However, this finding could not be used to differentiate AVM from haemangioma. Arterial-lesion enhancement times were shorter than 5 s in all AVMs and in 53% of haemangiomas but none of the venous malformations revealed arterial-lesion enhancement time of ≤5 s. Thus, arterial-lesion enhancement time is not a significant factor in differentiating high-flow from low-flow vascular malformations, contrary to findings reported by van Rijswijk CS et al., [17]. However, absence of early arterial enhancement helps in diagnosis of venous malformation.
Furthermore, a contrast rise time of <10 s can be used to differentiate AVM from other vascular anomalies, Ohgiya Y et al., proposed that the contrast rise time of lesions was shorter in high-flow malformations than in low-flow malformations, and no overlap was observed between the two groups because it can reflect the haemodynamics of vascular lesions [19]. The results of present study agree with that of Oghiya Y et al., [19].
In this study, attempts were made to assess the intra-observer reliability of US and CEMRI in characterising vascular anomalies, considering histopathology as the gold standard. To the best of our knowledge, this has not been done in previous literatures. A similar (near perfect) level of similarity was observed using both US and CEMRI, but was slightly higher in the latter. From the result of the present findings, it is conceivable that both US and CEMRI are ideal and accurate imaging modalities for characterisation of soft tissue vascular anomalies.
Furthermore, given that vascular anomalies predominantly affect paediatric patients, US may be preferred, particularly in patients where contrast administration is contraindicated or not recommended, particularly those with history of contrast allergy or renal failure.
Limitation(s)
As US is operator dependent, the expertise and experience of the radiologist influences the introduction of errors in the final diagnosis. In addition, we had a single centre design and the sample size of thef study was small, thus a similar study with larger cohort is recommended to confirm the findings of the present study. Use of US contrast could have improved visualisation of vascular architecture in real time and may be utilised in future similar comparative studies.
Conclusion(s)
Both US and CEMRI are highly precise in detection and characterisation of soft tissue vascular anomalies showing comparable level of agreement. Given the similar level of agreement, US may be preferred as it is widely available, cost and time sensitive and non invasive. Furthermore, evaluation of vascular characteristics on Colour Doppler, particularly flow velocity, is highly sensitive in differentiation of AVM and haemangiomas and does not require intravenous contrast. CEMRI may be reserved as a complementary technique in cases with diagnostic ambiguity in lesion characterisation or extent.