Multiple Myeloma (MM) is characterised by clonal B cell proliferation affecting elderly age group and involving various organ systems namely haematological, renal and skeletal system. Kidney may be affected in 50% of cases of MM. Though, Chronic Kidney Disease (CKD) is usually seen in MM, unusual presentations have been documented. The present case is of a 50-year-old female with complains of breathlessness and vague generalised symptoms. Routine tests showed blood urea level of 90 mg/dL and serum creatinine of 8.3 mg/dL. Further investigations revealed 24 hour protein level 48.5 gm/day, Erythrocyte Sedimentation Rate (ESR)-126 mm/1st hour, cast nephropathy in renal biopsy. Immunohistochemical (IHC) study on renal biopsy revealed kappa light chain deposits in tubules, raised kappa light chains (3280.00 mg/L) in serum Free Light Chain (FLC) assay and MM in bone marrow aspiration and biopsy study. So, MM should be considered in differential diagnosis in elderly patients presenting with acute severe renal failure.
Acute kidney injury, Immunoglobulin kappa chain, Multiple myeloma
Case Report
A 50-year-old female presented with breathlessness since two months, bilateral knee joint pain, vague back pain since one year. She had no history of fever/ rash/ oedema/ oral ulcer/ any urinary complaints/ Coronary Artery Disease (CAD)/ liver disease/ bad obstetric history. She had attained menopause 12 years back. Patient was hypertensive and was on medication for that. Family and social history were not significant. On physical examination, her pulse was 80/minutes, blood pressure was 118/80 mm of Hg. She had mild pallor and bilateral pitting pedal oedema. She had no icterus, cyanosis, clubbing, lymphadenopathy. Per abdomen examination was soft and no organomegaly was found.
Detailed routine investigations showed serum urea-90 mg/dL, serum creatinine-8.3 mg/dL, 24 hour urine protein level-48.5 gm/day, urine Albumin Creatinine Ratio (ACR)-583.2 mg/gm, spot urine albulmin-4.48 mg/dL, spot urine creatinine-7.9 mmol/day, serum sodium-134 mEq/L, serum potassium-3.5 mEq/L, serum calcium-9.6 mg/dL, serum phosphorus-6.5 mg/dL, serum uric acid-5.0 mg/dL, serum albumin-4.2 gm/dL, serum total protein-6.8 gm/dL, Serum Glutamic Oxaloacetic Transaminase (SGOT)-45 U/L, Serum Glutamic-Pyruvic Transaminase (SGPT)-10 U/L, Gamma-Glutamyl Transferase (GGT)-24.81 U/L, C3-140 mg/dL, C4-28 mg/dL, p Anti Neutrophil Cytoplasmic Antibody (ANCA)-0.14 AU/mL, cANCA-0.05 AU/mL, HIV/HbSAg/HCV-negative, serum amylase-69 U/L, serum lipase-84 U/L, Troponin I-0.012 μG/L, Haemoglobin-11.2 gm/dL, Total Leukocyte Count-12300/cmm, Total Platelet Count-2.47 lac/cmm, Erythrocyte Sedimentation Rate (ESR)-126 mm/1st hour, Prothrombin Time (PT)-9.2 sec, INR-1.14, activated partial thromboplastin time (aPTT)-29.3 sec. Urine routine microscopy revealed 2+Albuminuria, urine pus cells: 10-15/hpf, Red Blood Cells (RBCs):2-4/hpf, Granular casts: 0-2/LPF. Renal ultrasound showed right kidney measuring 12.9×6.5×7.4 cm and left kidney measuring 11.4×6.1×6.2 cm. There was no evidence of any mass lesions, hydronephrosis, or calcifications. On next four consecutive days repeat serum urea were 98, 101, 110 and 117 mg/dL, respectively and serum creatinine were 8.6, 8.8, 8.9 and 9.4 mg/dL. Urine Culture and Sensitivity showed no growth. Bence jones protein test was negative. Due to persistently raised serum urea and creatinine level and raised 24 hr urine protein level as well as raised urine ACR patient was advised for renal biopsy. Ultrasonography (USG) guided percutaneous kidney biopsy was done for Light Microscopic (LM) and Direct Immunofluorescence (DIF) studies.
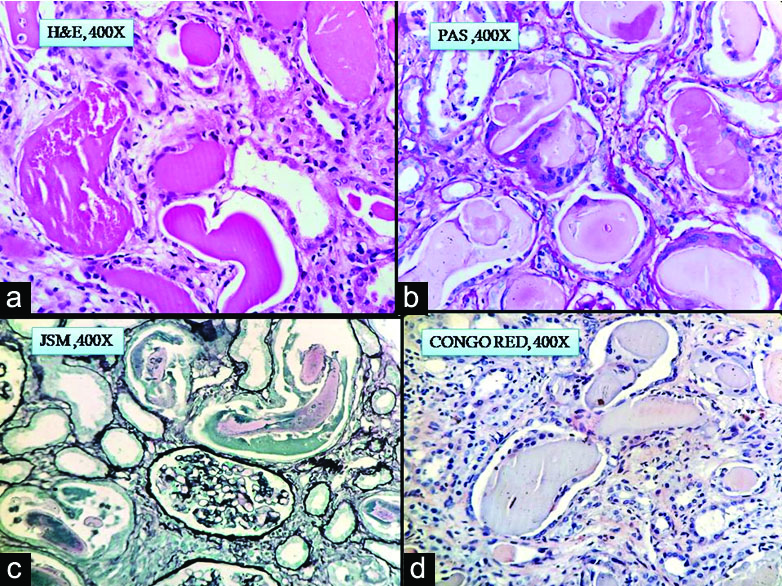
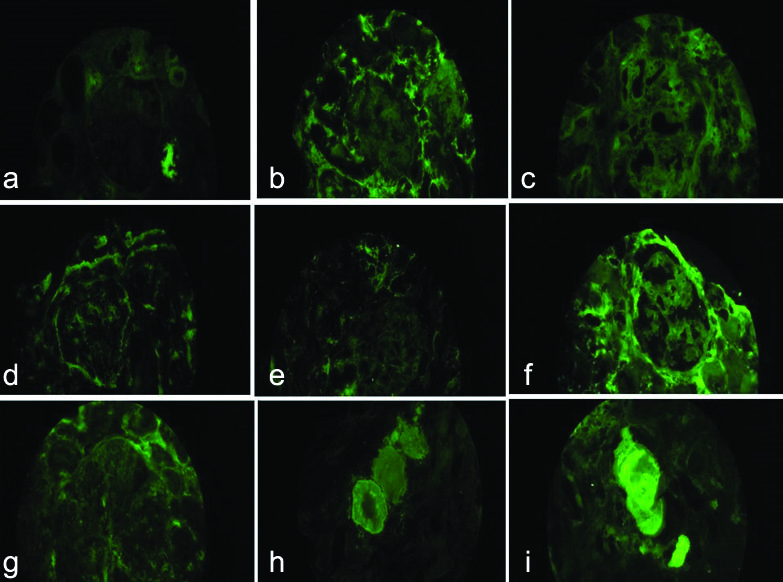
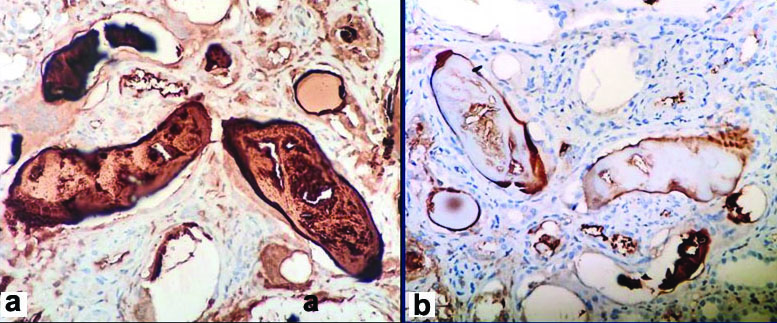
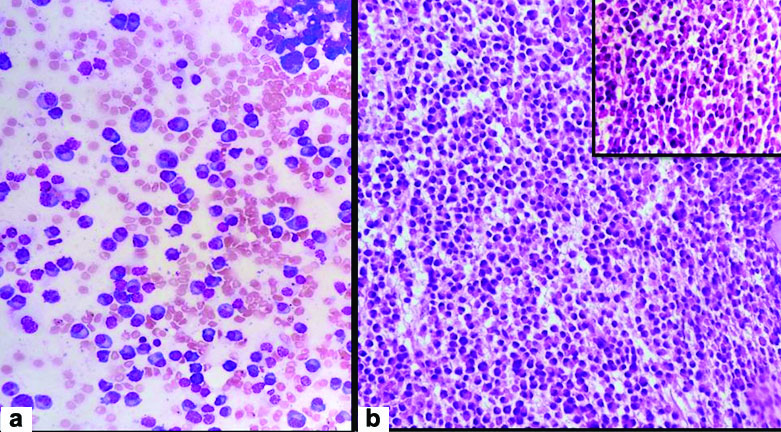
Clinical diagnosis was rapidly progressive renal failure with provisional diagnosis of Pauci immune Crescentic Glomerulonephritis or Immune complex mediated Crescentic Glomerulonephritis or Acute interstitial nephritis. Histopathology section showed a single core of renal tissue containing 14 glomeruli and surrounding tubules, interstitium and blood vessels. The glomeruli were normal in morphology [Table/Fig-1a,b]. Many tubules were dilated, filled with casts with foreign body giant cell reaction around them. The casts had fractured appearance and were Periodic Acidic Schiff (PAS) and Jones Silver Methenamine (JSM) stain negative. Congo red stain was done to rule out amyloidosis as the deposits were amorphous, acellular and eosinophilic. It was negative [Table/Fig-2a-d]. DIF against IgG, IgA, IgM,C3,C1q, fibrinogen were negative. Kappa and lambda on DIF showed positivity in tubular deposits [Table/Fig-3a-i]. In IHC study of biopsy kappa was positive, but lambda was negative in tubular deposits [Table/Fig-4a,b]. Serum FLC assay revealed κ-3280.00 mg/L (3.30-19.40 mg/L), lambda-13.40 mg/L (5.71-26.30 mg/L) and greatly raised κ: lambda ratio -244.776 (0.26-1.65). Serum β2 microglobulin level was 24,000 mg/L. So to rule out possibility of plasma cell disorder bone marrow study was advised. Bone marrow aspiration smears were hypercellular with suppression of erythropoiesis, myelopoiesis and megakaryopoiesis. These haematopoietic elements were replaced by immature plasma cells constituting >90% of marrow nucleated cells. Bone marrow biopsy showed hypercellular marrow with sheets of neoplastic plasma cells [Table/Fig-5a,b]. X-ray skull showed presence of multiple punched out lytic lesions. So final histopathological diagnosis was Light chain Cast nephropathy with Tubulointerstitial nephritis (MM associated). Patient was treated with bortezomib for MM. After 3rd dose she developed haematemesis and was on ventilator support for two days, but did not survive.
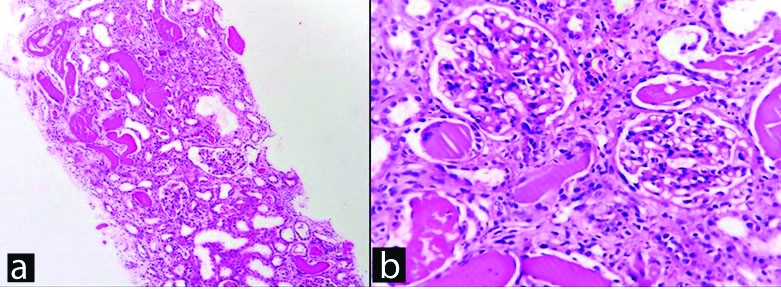
a) Renal biopsy, H&E stain, X100. b) Normal glomeruli, H&E stain, X400.
a-c) Fractured casts with giant cell reaction in H&E, PAS, JSM stain, X400; d) Tubular deposits negative (Congo red stain, X400).
a-c) IgA, IgG, IgM negative in glomeruli (DIF, X400); d-g) C3,C1q, Albumin, Fibrinogen negative in glomeruli (DIF, X400); h-i) Kappa and lambda positive in tubular deposits (DIF, X400).
a) Kappa positive in tubular deposit (IHC, X400); b) Lambda negative in tubular deposit (IHC, X400).
a) Immature plasma cells >90% in bone marrow aspiration smear (H&E stain, X400); b)Hypercellular marrow in bone marrow biopsy section (H&E stain, X100), (Sheets of neoplastic plasma cells H&E stain, X400, inset)
Discussion
MM presents with renal failure in 20%-50% cases [1]. Renal involvement in MM may be as Acute Kidney Injury (AKI) or Nephrotic Syndrome (NS) or CKD [2]. Renal pathology in MM show plethora of changes affecting all the compartments of kidney either in isolation or in combination. It may affect the tubules as cast nephropathy (40–63%) and proximal tubulopathy, may involve the glomeruli in the form of Monoclonal Immune Deposition Disease (MIDD) (2-3%), amyloidosis (4-5%), Membranous Glomerulonephritis (MGN), Membranoproliferative Glomerulonephritis (MPGN), Thrombotic Microangiopathy (TMA) and less commonly cryoglobulinemic renal disease (<1%), or may present as Acute Tubulointerstitial Nephritis or plasmacytoma involving tubulointerstitium [3-5].
Cast nephropathy is observed in almost half of cases of MM. When circulating excess toxic monoclonal light chain proteins reach kidney to be filtered, functional capability of kidney is overwhelmed, leading to their impaired removal. These light chains are endocytosed and degraded in Proximal Convoluted Tubules (PCT) after filtration by glomerulus. The excess light chain overwhelms the catabolic capacity of the tubular cells also. So, they reach the distal tubules and form tubular casts by mixing with Tamm-Horsfall protein (uromodulin) obstructing the distal tubules. The fractured appearance in histology sections is due to the crystalline nature of light chains which makes these casts hard but brittle and during section cutting the casts gets teared up. Few precipitating factors enhance cast formation such as dehydration, acidosis, hypercalcaemia, use of nephrotoxic medications like Non Steroidal Anti-Inflammatory Drugs (NSAIDs), diuretics and Angiotensin-Conversing Enzyme Inhibitors (ACEI) [6-8]. The persistent tubular obstruction increases intratubular luminal pressure, reducing the interstitial blood flow and Glomerular Filtration Rate (GFR), affecting the renal function [2,9]. Also, when endocytosed by tubular epithelial cells, the light chains activate Nuclear factor kappa-light chain enhancer of activated B cells (NF-kB) and cause apoptosis of tubular epithelial cells or induce Epithelial-Mesenchymal Transition (EMT), leading to transcription of inflammatory cytokines and profibrotic factors. Consequent tubulointerstitial inflammation and fibrosis determines the overall prognosis of disease process. Though the rate of cast formation is directly proportional to the amount of light chains, the degree of toxicity depends upon the variable region of the light chain which determines its nephrotoxicity. This is why in some cases even small amounts of light chains may cause early renal function impairment even before appearance of other clinical manifestations of MM, whereas in others even large amounts may cause minimal renal dysfunction. Among lambda and kappa light chains, lambda light chains are more commonly associated with amyloidosis and kappa are known to be involved in other types of renal damage [8-10].
Peculiarities associated in index case are: 1) Acute presentation of case though MM usually presents as CKD; 2) Mild degree of anaemia though bone marrow was almost replaced by myeloma cells; 3) Lack of hypercalcaemia; 4) Disproportionately high degree of proteinuria and relatively low ACR; and 5) Lack of any glomerular involvement. Anaemia is very common association in patients with MM, but in present case patient had no significant anaemia. She also did not have hypercalcaemia despite having multiple destructive bone lesions. The high 24 hour urine protein level without any glomerular pathology can be explained by the fact that it was due to presence of both albumin and light chains in urine, the later component being the major contributor. A low microalbumin to creatinine ratio in spot urine specimen with a high total urine protein level is highly indicative of light chain proteinuria and should be confirmed with specific tests as the albumin excretion is not the dominant cause of proteinuria in this case and ACR is specific for microalbuminuria. Urine dipsticks detect albumin alone and so fail to detect myeloma paraproteins like index case [3]. Absence of these five features made index case a rare and unusual one. The morphological differential diagnosis for cast nephropathy are rhabdomyolysis associated casts, casts in End Stage Renal Disease (ESRD) and casts in mTor inhibitor toxicity. In all these conditions monoclonal light chain deposits in DIF study is not seen. Beside that, history of muscle injury or post-transplant set up helps in differentiation. Also, giant cell reaction surrounding the casts not seen in case of ESRD and mTor induced toxicity. As cast nephropathy develops in MM, the histological diagnosis may be straight forward but can be a challenge to establish when the underlying myeloma has not yet been suspected as happened in present case. In this scenario serum FLC assay, urine protein electrophoresis, Bence-Jones protein test, bone marrow study is extremely helpful like index case. The serum of kappa/lambda concentrations and ratio act as surrogate markers for monoclonality and high serum monoclonal FLC (>500 mg/L) is predictive of developing AKI [2,8].
Plasmapheresis and haemodialysis to remove excess FLC, chemotherapy with proteasome inhibitors like bortezomib and carfilzomib, immunomodulator therapy in the form of thalidomide and lenalidomide etc., good supportive care, use of bisphosphonates for hypercalcaemia, glucocorticoids and autologous stem cell transplantation are the modality of treatments available. Advancements in diagnostic methods, chemotherapeutic agents and dialysis techniques showing improved outcomes for MM patients with kidney disease [2,11].
Conclusion(s)
Cast nephropathy in myeloma may cause severe acute renal dysfunction leading to significant degree of morbidity and mortality even before diagnosis of MM. So it should always be considered in differential diagnosis of unexplained acute renal failure in middle aged and elderly persons. Proper clinical judgement, prompt diagnosis in suspicious cases with the help of ancilliary tests and rapid institution of appropriate therapy is essential for survival of patients in such scenarios and stop progression of the disease to CKD stage.
Author Declaration:
Financial or Other Competing Interests: None
Was informed consent obtained from the subjects involved in the study? No
For any images presented appropriate consent has been obtained from the subjects. No
Plagiarism Checking Methods: [Jain H et al.]
Plagiarism X-checker: Jun 06, 2020
Manual Googling: Jul 16, 2020
iThenticate Software: Aug 25, 2020 (10%)
[1]. Prakash J, Niwas SS, Parekh A, Vohra R, Wani IA, Sharma N, Multiple myeloma- Presenting as acute kidney injuryJAPI 2009 57:23-26. [Google Scholar]
[2]. Katagiri D, Noiri E, Hinoshita F, Multiple myeloma and kidney diseaseThe Scientific World Journal 2013 2013:48728510.1155/2013/48728524288486 [Google Scholar] [CrossRef] [PubMed]
[3]. Heher EC, Rennke HG, Laubach JP, Richardson PG, Kidney disease and multiple myelomaClin J Am Soc Nephrol 2013 8:2007-17.10.2215/CJN.1223121223868898 [Google Scholar] [CrossRef] [PubMed]
[4]. Soleymanian T, Soleimani A, Musavi A, Mojtahedi K, Hamid G, Outcome of patients with multiple myeloma and renal failure on novel regimensSaudi J Kidney Dis Transpl 2016 27(2):335-40.10.4103/1319-2442.17855726997388 [Google Scholar] [CrossRef] [PubMed]
[5]. Korbet SM, Schwartz MM, Multiple myelomaJ Am Soc Nephrol 2006 17:2533-45.10.1681/ASN.200602013916885408 [Google Scholar] [CrossRef] [PubMed]
[6]. Huang ZQ, Sanders PW, Biochemical interaction between Tamm-Horsfall glycoprotein and Ig light chains in the pathogenesis of cast nephropathyLab Invest 1995 73:810-17. [Google Scholar]
[7]. Sanders PW, Booker BB, Pathobiology of cast nephropathy from human Bence Jones proteinsJ Clin Invest 1992 89:630-39.10.1172/JCI1156291737851 [Google Scholar] [CrossRef] [PubMed]
[8]. Hutchison CA, Batuman V, Behrens J, Bridoux F, Sirac C, Dispenzieri A, The pathogenesis and diagnosis of acute kidney injury in multiple myelomaNature Reviews Nephrology 2012 8(1):43-51.10.1038/nrneph.2011.16822045243 [Google Scholar] [CrossRef] [PubMed]
[9]. Corso A, Zappasodi P, Lazzarino M, Urinary proteins and renal dysfunction in patients with multiple myelomaBiomed Pharmacother 2002 56:139-43.10.1016/S0753-3322(02)00171-3 [Google Scholar] [CrossRef]
[10]. Dimopoulos MA, Kastritis E, Rosinol L, Blade J, Ludwig H, Pathogenesis and treatment of renal failure in multiple myelomaLeukemia 2008 22:1485-93.10.1038/leu.2008.13118528426 [Google Scholar] [CrossRef] [PubMed]
[11]. Yadav P, Cook M, Cockwell P, Current trends of renal impairment in multiple myelomaKidney Dis 2015 1:241-57.10.1159/00044251127536684 [Google Scholar] [CrossRef] [PubMed]