Introduction
Anti-RR antibodies have recently been described as cytoplasmic pattern in immunofluorescence-based screening of autoantibodies on Hep-2 cells [1]. Recently, the ICAP has introduced this pattern in their reporting guideline for Antinuclear Antibodies (ANA) and has described this as “distinct RR structures in cytoplasm of interphase cells”. The ICAP has given it a reporting code of Anti-Cellular-23 (AC-23) pattern [1].
Among several ANA patterns described, some are specific for a particular autoimmune condition while others occur randomly in individuals with or without AD. The RR pattern comprises of fibrillary structures resembling rods (3-10 μm) and rings (2-5 μm) in the cytoplasm. Molecular studies have found two target enzymes, Inosine Monophosphate Dehydrogenase type-2 (IMPDH2) and Cytidine Triphosphate Synthase Type 1 (CTPS1), associated with this pattern [2]. Earlier studies have described its association exclusively with ribavirin and/or INF-α therapy given for hepatitis C [3]. Later clinical relevance was observed in the terms of poor response to therapy and increased liver fibrosis [2-6]. A few recent reports have found RR pattern in non Hepatitis C Virus (HCV) patients and healthy individuals as well [5,7].
In light of importance of RR-ANA pattern, we searched our archive of ANA reports (routine clinical sample for ANA test) for RR pattern from May 2019 to January 2020 retrospectively and encountered with eight such cases after analysing 25,242 ANA reports. Further these cases were traced back to find its clinical association. Immunological and clinical database were hunted by Immunopathologist (expert in ANA reporting) and clinician respectively to find RR positive with HCV negative cases along with their clinical association. Three patients were positive on HCV serology, however other five patients were negative. Routinely, ANA screening in our lab is being done on Hep-2 cell line provided by NOVA-Lite, however during retrospective analysis these cases were reassessed with another commercially available substrate (Euroimmun, Hep-2 cell line). Retrospective analysis of clinical data helped us to reach clinical association in these five cases which have been described in [Table/Fig-1].
Major characteristics of HCV negative patients with rods and rings Anti-Nuclear Antibody (ANA) pattern.
| Case No. | Age/Sex | Major clinical presentation | Haemogram | LFT* | ANA pattern | History of poly-pharmacy | Relevant other specific tests | Diagnosis |
|---|
| 1. | 50/F | Joint pain especially smaller joint of hand, Malaise | Hb: 13.9 gm/dLTLC: 9.9×109/LDLC: N39, L46, M14, E01Platelets: 289×109/L | ALP -186 U/LALT, AST, albumin and bilirubin- Within normal limit | +3 RR | Yes | Anti-CCP positive | Rheumatoid arthritis |
| 2. | 36/M | Easy fatigability, loose stool and abdominal discomfort | Hb: 14.93 gm/dLTLC: 5.8×109/LDLC: N68, L24, M06, E01, B01Platelets: 104×109/L | All within normal limit | +3 RR | Yes | -- | Idiopathic Thrombocytopenia with associated non-specific gastrointestinal upset |
| 3. | 15/F | Abdominal pain, Diarrhoea, low grade fever, weakness | Hb: 9.94 gm/dLTLC: 6.4×109/LDLC: N53, L31, M12, E03, B01Platelets: 518×109/L | ALP -172 U/LALT, AST, albumin and bilirubin- Within normal limit | +3 RR | Yes | Anti-IgG for Amoebiasis | Amoebiasis, Moderate iron deficiency anaemia |
| 4. | 37/M | Fever, anorexia, malaise, nausea and right hypochondrial discomfort | Hb: 8.3 gm/dLTLC: 6×109/LDLC: N72, L21, M05, E02Platelets: 283×109/L | ALP-80 U/L; ALT-948 U/L; AST- 675 U/LAlbumin-within normal limitRaised Conjugated bilirubin | +3 RR | No | Hepatitis B Positive | Hepatitis B |
| 5. | 28/F | On-off bluing and ulceration of finger tips and toe after exposure to cold | Hb: 10.3 gm/dLTLC: 6.7×109/LDLC: N72, L24, M03, E01Platelets: 243×109/L | All within normal limit | +3 RR and few nuclear dots | Yes | ANA immunoblot CENP-B=+2Liver blot-S100=+3 | Possibly undifferentiated connective tissue disease (with Raynaud’s) |
{LFT* Liver function tests include; ALP: Alkaline phosphatase, ALT: Alanine transaminase, AST: Aspartate aminotransferase, albumin, bilirubin, Hb: Haemoglobin, TLC: Total leucocyte count, DLC: Differential leukocyte count, RR: Rods and rings, ANA: Anti-nuclear antibody, Anti-CCP: Anti-cyclic citrullinated peptide, N: Neutrophils, L: Lymphocytes, M: Monocytes, E: Eosinophill, B: BAsophill, IgG-Immunoglobulin G, CENP-B: Centromere protein B}
Case 1
A 50-year-old female presented to Out Patient Department (OPD) with joint pain (smaller joints of hand) getting worse in morning and non-specific generalised malaise. Routine haemogram and ANA testing was asked for which normal haemogram and positive anti-Cyclic Citrullinated Peptide (anti-CCP) and cytoplasmic RR pattern on ANA [Table/Fig-2a]. Liver Function Test (LFT) and viral markers were enquired. Alkaline Phosphatase (ALP) was raised however other LFT were normal and serology for HCV was negative [Table/Fig-1]. Patient was diagnosed as RA and was put on analgesics. During retrospective analysis, patient was asked for follow-up (after three months) and repeat HCV serology and ANA testing with two commercial substrates (NOVA-Lite and Euroimmun) were performed. ANA revealed RR pattern and HCV serology was again negative. On follow-up, patient complained of persistence of malaise along with anorexia and easy fatigability. Repeat haemogram with Thyroid Function Test (TFT), vitamin-B12, vitamin D and lipid profile along with cardiac work up were requested and all were within normal limit. Patient was continued on analgesics with addition of nutritional supplement and is doing well till last follow-up.
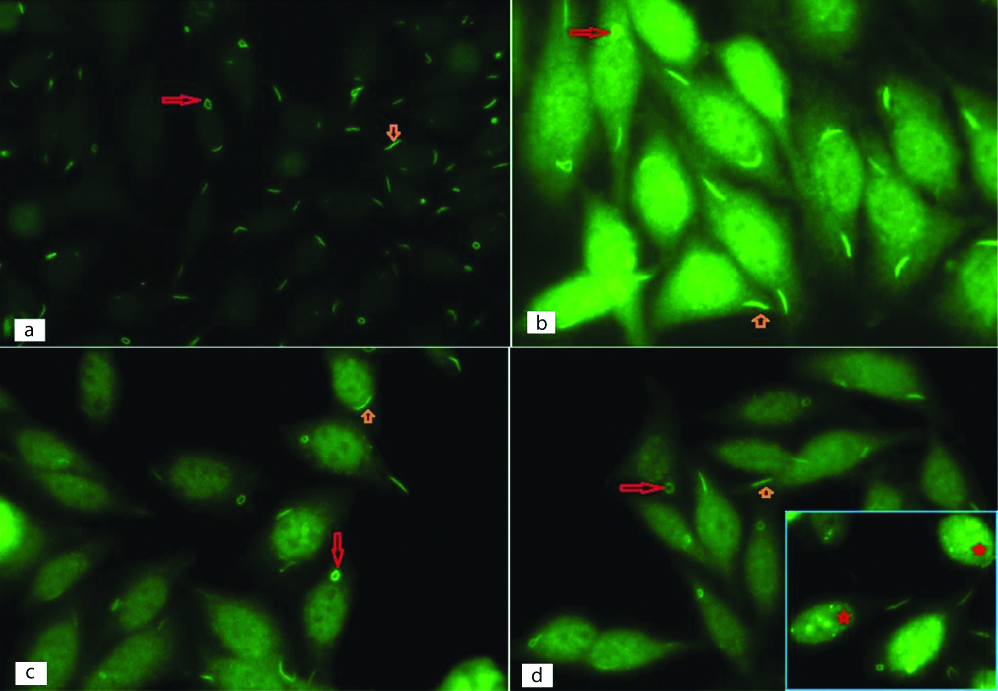
Rods and rings antinuclear antibody pattern on indirect immunofluorescence (IIF) in non-hepatitis C patients on slides of HEp-2 (Human Epithelial type 2) cells. Rods (small orange arrow) and rings (long red arrow) in cytoplasm of HEp-2 cells are seen {(a-Case -1, 40x); (b-Case 2, 100x); (c-Case 3,100x); (d-Case 4,100x) (d-‘Inset’ shows few nuclear dots (red star) along with RR in case-5, 100x)}.
Case 2
A 36-year-old male presented with easy fatigability, loose stool and abdominal discomfort for duration of two months. He had past history of haemorrhoid treatment and local medication for his fatigability. His blood counts were normal except thrombocytopenia [Table/Fig-1]. The lipid profile, gastrointestinal endoscopy, enteric biopsy, stool examinations and cardiac workup were within normal limits. Patient was put on anti-spasmodic and asked to follow-up after four months. Abdominal symptoms subsided however in view of persistent thrombocytopenia, ANA and TFT were done. Indirect immunofluorescence revealed a cytoplasmic RR-ANA pattern [Table/Fig-2b] and TFT was normal. To exclude possibility of Hepatitis C, HCV serology and LFT were enquired and both turned out to be unremarkable. In view of above observations diagnosis of Idiopathic Thrombocytopenia (ITP) was made. Later patient was asked to come after three months and repeat testing for ANA and HCV serology showed similar results (HCV negative, ANA positive with RR pattern).
Case 3
A 15-year-old, very sick and emaciated young female presented with low-grade fever, diarrhea, abdominal pain and weakness for 2-3 weeks. Routine haemogram revealed moderate anaemia. It was utmost important to know cause of anaemia in this critically ill patient. So, urgent iron profile and haemolytic workup {including ANA and direct coombs test to exclude Systemic Lupus Erythematosus (SLE)} were asked for. Haemolytic workup was unremarkable however iron deficiency was quite evident on iron profiling and ANA revealed RR pattern [Table/Fig-2c]. Simultaneously, stool examination highlighted amoebic trophozoites however anti-tissue transglutaminase antibody (for celiac disease) and serology for malaria and dengue were negative. Positive IgG antibody for Entamoeba histolytica supported our diagnosis however, also compelled us to rule out liver involvement. LFT and abdominal ultrasonography were unremarkable except raised ALP. In light of above clinical and laboratory parameters, diagnosis of amoebiasis with moderate anaemia was made [Table/Fig-1]. Later during retrospective evaluation, HCV serology and dsDNA were done on stored sample for academic interest and found to be negative.
Case 4
A 37-year-old male patient presented with viral syndrome like features including fever, anorexia, fatigue, malaise, nausea and right hypochondrial discomfort. Haemogram and LFT were performed, revealing moderate anaemia and raised ALT (948 U/L) and AST (675 U/L). To explore cause of hepatitis, viral serology and Autoimmune Hepatitis (AIH) workup (including ANA testing) were done. Serology for hepatitis B virus was positive however HCV and Hepatitis A Virus (HAV) were negative. ANA testing highlighted RR pattern [Table/Fig-2d] however, AIH immunoblot was negative. Diagnosis of hepatitis B was made and anti-viral therapy was prescribed accordingly.
Case 5
A 28-year-old female presented with on and off discolouration, bluing and ulceration of fingertip for last 5-6 years which got worse in winter/cold. Intermittently, she took local medications (complementary and alternative medicine) for long time. Routine haemogram revealed mild anaemia however ANA test (to rule out autoimmune aetiology) highlighted few nuclear dots along with cytoplasmic RR pattern ([Table/Fig-2d], Inset). In view of RR positivity, HCV serology and LFT were performed [Table/Fig-1]. LFT was within normal limit however HCV serology was borderline positive which later confirmed as negative through molecular test (viral RNA). In light of few nuclear dots, ANA and AIH immunoblot were performed to explore possible antigen responsible for this pattern and highlighted positivity for Centromere Protein B (CENP-B) (+2) and S100 (+3). She was labelled as possible undifferentiated connective tissue disease (with Raynaud’s).
Discussion
ANA testing through IIF is required to look for autoantibodies in different diseases specially AD. Out of several ANA patterns, RR is a recently introduced and is relatively unexplored pattern, with a lack of reports on its clinical associations. Since its first observation, RR has exclusively been associated with ribavirin/INF-α treatment in hepatitis C patients (15-30% of hepatitis C with ribavirin/INF-α treatment) [5,8]. In present study, eight cases of RR pattern were found out of 25,242 clinical samples examined. Among these only three patients had HCV infection and ribavirin/INF-α therapy, however others didn’t. The diagnosis of HCV was based on serology which has diagnostic limitation in window period (time taken for seroconversion). To deal with this issue patients were asked to come after three months interval from first test. Three came for follow-up and their HCV serology was again negative however one patient didn’t turn up for follow-up. Viral RNA for HCV was already negative in fifth case leaving behind need of repeat testing.
Recognition of this pattern has already attracted many researchers who are trying to explore the pathobiology behind its generation and clinical correlates [2-4]. Molecular studies have revealed two enzymatic targets IMPDH2 and CTPS1, which are involved in purine metabolism and protein aggregation phenomenon [6,8]. It has also been observed that ribavirin causes IMPDH2 inhibition leading to cellular alteration and cytotoxicity, which is further enhanced by immunomodulatory function of INF. This hypothesis has been proposed as a possible pathway of RR antibody generation in HCV treatment associated RR antibody [8]. Recently, it has been demonstrated that inhibitors of these two enzymes in the form of drug (mycophenolic acid and ribavirin), promote RR antibody formation [2,9]. The ex-vivo experiments have also identified other metabolites/drugs (glycine, dihydrofolate reductase inhibitor, deprivation of serine), which may be inducer of RR antibody production, directly or indirectly through pathways involved in nucleotides biosynthesis [9,10]. Functional significance of RR is still a matter of debate. Few observations hypothesised that RR formation (enzyme aggregates) could be an important regulatory mechanism of enzyme activity involved in Guanosine Triphosphate/ Cytidine Triphosphate (GTP/CTP) biosynthesis [11,12]. However, it seems that there is involvement of multiple other molecules/pathways, which are affected or regulated by various drugs leading to anti-RR antibodies formation.
In recent years, various ex-vivo and population-based studies have been conducted to explore its clinical relevance. However, only limited literature is available describing RR-ANA pattern in non-hepatitis C human subjects [Table/Fig-3] [5,7,13,14]. It has been observed that like other ANA patterns, RR pattern may also be seen in healthy individuals representing general population [7]. In a large population-based study conducted by National Health and Nutrition Examination Survey (NHANES) on 4,738 individuals, 39 individuals were found to be positive for anti-RR antibodies and only one had history of earlier HCV. In addition, 79 other individuals were incidentally found to be positive for HCV and all were negative for RR-ANA pattern, suggesting lack of significant association between RR pattern and hepatitis C [7]. In another retrospective analysis of 22,915 clinical samples, 87 anti-RR positive cases were reported, out of which 73 (84% of anti-RR positive) were HCV positive, however others (14/87) were negative. These non-HCV cases had various presentation/s including bronchial asthma, RA, Wilson’s disease, Wegener’s Granulomatosis (WG), SLE, Chronic Obstructive Pulmonary Disease (COPD), psoriasis, hypertension, dyslipidemia and Goodpasture’s disease [5]. One recent case report demonstrated RR pattern in a child with bronchopneumonia and chronic lung disease who later was discharged after excluding cystic fibrosis, HCV and Human Immune Deficiency Virus (HIV) [13]. Keppeke GD et al., screened defined disease population (397 HCV positive and 200 non-HCV patients) for RR pattern and could find only one RR positive case in non-HCV group which was a case of hepatitis B [14]. Clinical relevance of RR antibody is still debatable as some of the studies report its positive correlation with higher incidence of relapse and liver fibrosis while others didn’t find any clinical relevance [3,5,14,15]. In our record, only one of the three HCV patients had relapse.
Summary of studies documenting association of rods and ring ANA pattern in non-HCV patients [5,7,13,14].
| S. No. | Study | Sample type | Number of RR positive cases | HCV and RR both positive | HCV negative and RR positive | Diagnosis/Diseases association of non hepatitis C cases |
|---|
| 1. | Climent J et al., [5] | 22,915, Clinical samples for routine ANA screening | 87 | 73 | 14 (HCV serology was available in 10 cases only) | Bronchial asthma, Wilson’s disease, Nephropathy, RA, Primary antiphospholipid syndrome, Psoriasis, Ictus, Wegener’s granulomatosis, COPD, Cholangitis, Polyarthralgia, SLE, Porto-mesenteric venous thrombosis and Goodpasture’s disease |
| 2. | Shaikh Y et al., [7] | 4,738, Apparently healthy general population | 39 | 01 | 38 | No disease as such however significant number of persons have history of polypharmacy intake for non-specific ailment/s. |
| 3. | Magerl M et al., [13] | One case report | -- | -- | 01 | Bronchopneumonia and chronic lung disease |
| 4. | Keppeke GD et al., [14] | 597, Defined disease subjects {397 HCV and 200 non-HCV (non-HCV included SLE, Systemic sclerosis, polymyositis, multiple sclerosis, hepatitis B and autoimmune hepatitis)} | 57 | 56 | 01 | Hepatitis B |
| 5. | Present study | 25,242 clinical samples for routine ANA screening | 08 | 03 | 05 | RA, Idiopathic Thrombocytopenia, Amoebiasis with Iron deficiency anaemia, Hepatitis B, Possibly undifferentiated connective tissue disease (with Raynaud’s) |
ANA: Anti-nuclear antibody; HCV: Hepatitis C virus; RA: Rheumatoid arthritis; COPD: Chronic obstructive pulmonary disease; SLE: Systemic lupus erythematosus
In the present series, all the five patients did not have history of HCV or associated treatment and clinically they had different spectrum of disease: RA, amoebiasis, possible undifferentiated connective tissue disease (with associated Raynaud’s), hepatitis B and ITP (with associated gastrointestinal upset). Four patients (RA, Raynaud’s, amoebiasis and ITP) had taken multiple medications. History of poly-pharmacy seems to be the most significant factor associated with anti-RR antibody generation supported by available literature [7]. Apart from these, some data has claimed that its detection is substrate specific, as it has not been identified uniformly with all commercial substrates [16]. In present series, ANA testing was performed on two commercially available substrates (NOVA-lite and Euroimmun) displaying similar RR pattern. So, issue of commercial substrate is unlikely to be in present series.
Conclusion(s)
RR-ANA pattern is not restricted to hepatitis C and associated treatment (ribavirin/interferon). It may be detected in various autoimmune conditions (RA, undifferentiated connective tissue diseases) and other diseases like ITP, amoebiasis and hepatitis B. Exact inciting factor and pathogenesis of RR-ANA pattern is still a mystery, however poly-pharmacy appears to be most convincing aetiological factor till date. This is an evolving area and it is important to document all clinical associations to have a good understanding of its significance.
{LFT* Liver function tests include; ALP: Alkaline phosphatase, ALT: Alanine transaminase, AST: Aspartate aminotransferase, albumin, bilirubin, Hb: Haemoglobin, TLC: Total leucocyte count, DLC: Differential leukocyte count, RR: Rods and rings, ANA: Anti-nuclear antibody, Anti-CCP: Anti-cyclic citrullinated peptide, N: Neutrophils, L: Lymphocytes, M: Monocytes, E: Eosinophill, B: BAsophill, IgG-Immunoglobulin G, CENP-B: Centromere protein B}
ANA: Anti-nuclear antibody; HCV: Hepatitis C virus; RA: Rheumatoid arthritis; COPD: Chronic obstructive pulmonary disease; SLE: Systemic lupus erythematosus
[1]. Chan EK, de Melo Cruvinel W, Andrade LE, The international consensus on standardized nomenclature of antinuclear antibody HEp-2 cell patterns (ICAP) initiative-Current state and perspectives. In 12thDresden Symposium on Autoantibodies 2015 Dresden, GermanyPabst Science Publishers:282-288. [Google Scholar]
[2]. Carcamo WC, Satoh M, Kasahara H, Terada N, Hamazaki T, Chan JY, Induction of cytoplasmic rods and rings structures by inhibition of the CTP and GTP synthetic pathway in mammalian cellsPloS one 2011 6(12)10.1371/journal.pone.002969022220215 [Google Scholar] [CrossRef] [PubMed]
[3]. Covini G, Carcamo WC, Bredi E, von Muhlen CA, Colombo M, Chan EK, Cytoplasmic rods and rings autoantibodies developed during pegylated interferon and ribavirin therapy in patients with chronic hepatitis CAntivir Ther 2012 17(5):805-11.10.3851/IMP199322293655 [Google Scholar] [CrossRef] [PubMed]
[4]. Seelig HP, Appelhans H, Bauer O, Blüthner M, Hartung K, Schranz P, Autoantibodies against Inosine-5’-monophosphate dehydrogenase 2-characteristics and prevalence in patients with HCV-infectionClinical Laboratory 2011 57(9):753 [Google Scholar]
[5]. Climent J, Morandeira F, Castellote J, Xiol J, Niubó J, Calatayud L, Clinical correlates of the “rods and rings” antinuclear antibody patternAutoimmunity 2016 49(2):102-08.10.3109/08916934.2015.111876226699543 [Google Scholar] [CrossRef] [PubMed]
[6]. Stinton LM, Myers RP, Coffin CS, Fritzler MJ, Clinical associations and potential novel antigenic targets of autoantibodies directed against rods and rings in chronic hepatitis C infectionBMC Gastroenterology 2013 13(1):5010.1186/1471-230X-13-5023506439 [Google Scholar] [CrossRef] [PubMed]
[7]. Shaikh Y, Krantz A, El-Farra Y, Anti-rods and rings autoantibodies can occur in the hepatitis c-naïve populationJournal of Preventive Medicine and Hygiene 2013 54(3):175 [Google Scholar]
[8]. Keppeke GD, Calise SJ, Chan EK, Andrade LE, Anti-rods/rings autoantibody generation in hepatitis C patients during interferon-α/ribavirin therapyWorld Journal of Gastroenterology 2016 22(6):196610.3748/wjg.v22.i6.196626877604 [Google Scholar] [CrossRef] [PubMed]
[9]. Calise SJ, Keppeke GD, Andrade LE, Chan EK, Anti-rods/rings: A human model of drug-induced autoantibody generationFrontiers in Imunology 2015 6:4110.3389/fimmu.2015.0004125699057 [Google Scholar] [CrossRef] [PubMed]
[10]. Calise SJ, Purich DL, Nguyen T, Saleem DA, Krueger C, Yin JD, ‘Rod and ring’ formation from IMP dehydrogenase is regulated through the one-carbon metabolic pathwayJ Cell Sci 2016 129(15):3042-52.10.1242/jcs.18340027343244 [Google Scholar] [CrossRef] [PubMed]
[11]. Noree C, Monfort E, Shiau AK, Wilhelm JE, Common regulatory control of CTP synthase enzyme activity and filament formationMolecular Biology of the Cell 2014 25(15):2282-90.10.1091/mbc.e14-04-091224920825 [Google Scholar] [CrossRef] [PubMed]
[12]. Keppeke GD, Andrade LE, Grieshaber SS, Chan EK, Microinjection of specific anti-IMPDH2 antibodies induces disassembly of cytoplasmic rods/rings that are primarily stationary and stable structuresCell & Bioscience 2015 5(1):110.1186/2045-3701-5-125601894 [Google Scholar] [CrossRef] [PubMed]
[13]. Magerl M, Gandra RF, Menolli RA, ANA-Hep2 Positive with anti-rods and rings pattern in a child without hepatitis c under treatment: Case reportJ Rheum Dis Treat 2019 5:07310.23937/2469-5726/1510073 [Google Scholar] [CrossRef]
[14]. Keppeke GD, Nunes E, Ferraz ML, Silva EA, Granato C, Chan EK, Longitudinal study of a human drug-induced model of autoantibody to cytoplasmic rods/rings following HCV therapy with ribavirin and interferon-αPloS One 2012 7(9)10.1371/journal.pone.004539223028980 [Google Scholar] [CrossRef] [PubMed]
[15]. Novembrino C, Aghemo A, Ferraris Fusarini C, Maiavacca R, Matinato C, Lunghi G, Interferon-ribavirin therapy induces serum antibodies determining ‘rods and rings’ pattern in hepatitis C patientsJournal of Viral Hepatitis 2014 21(12):944-49.10.1111/jvh.1228125040504 [Google Scholar] [CrossRef] [PubMed]
[16]. Francescantonio PL, Cruvinel WD, Dellavance A, Andrade LE, Taliberti BH, Von Mühlen CA, IV Brazilian guidelines for autoantibodies on HEp-2 cellsRevistabrasileira de Reumatologia 2014 54(1):44-50.10.1016/j.rbr.2013.10.001 [Google Scholar] [CrossRef]