Drug resistant malaria represents the most challenging health problem in a developing countries like India. In 2016, an estimated 216 million cases of malaria occurred worldwide, most of which, were in the African Region (90%), followed by the South-East Asia Region (7%). The incidence of malaria in India accounted for 58% of cases in the South East Asia Region of World Health Organisation (WHO) [1]. At present, official figures for malaria in India indicate 0.7-1.6 million confirmed cases and 400-1000 deaths annually [2]. The major threat to malaria control worldwide is the increasing incidence of complicated and drug-resistance cases. Among the antimalarials, ‘chloroquine’, which was used as the first-line drug for treatment, became resistant and was responsible for the resurgence of malaria in the tropics and in Africa [3]. This led to the use of sulphamethoxazole-pyrimethamine combination therapy as the first-line drug, in treatment of acute uncomplicated malaria, which subsequently became resistant, contributing to a resurgence of malaria in tropical countries [4]. The new antimalarial drug “artimisinin”, discovered by Chinese Noble Laurette “TuYouyou”, has been used since the year 2001, in form of Artemisinin-based Combination Therapies (ACTs), and thus have become the cornerstone of the treatment of uncomplicated and complicated Plasmodium falciparum malaria, according to WHO [5]. Resistance to artemisinin has also emerged, as evidenced by retrospective analysis of molecular markers, which indicate that artemisinin resistance likely emerged in 2001, before the widespread deployment of ACTs in Cambodia, but significant clinical artemisinin resistance was only identified in 2006 [6]. Although multidrug resistance, including artemisinin (partial) resistance and partner drug resistance, has been reported in five countries of the Greater Mekong Subregion (GMS), there has been a massive reduction in malaria cases and deaths in this subregion, due to timely monitoring of drug efficacy [1]. The WHO defines resistance to antimalarials as “the ability of Plasmodium to survive and/or multiply despite the administration and absorption of a medicine given in doses equal to- or higher than- those usually recommended but within the tolerance of the subject”, with the subsequent statement that “the form of the drug active against the parasite must be able to gain access to the parasite or the infected red blood cell for the duration of the time necessary for its normal action” [7]. Artemisinin resistance is characterised by reduced susceptibility of the ring stage of parasite development [8] and is clearly associated with increasing rates of failure of artemisinin-based combination treatments in Cambodia and Thailand [9].
The term ‘artemisinin resistance’ is used to describe delayed parasite clearance observed after treatment with an artesunate monotherapy, or after treatment with an ACT [10]. The working definition of artemisinin resistance is based on clinical and parasitological outcomes observed during routine therapeutic efficacy studies of ACTs and clinical trials of artesunate monotherapy: an increase in parasite clearance time, as evidenced by ≥10% of cases with parasites detectable on day 3 after treatment with an ACT (suspected resistance) or treatment failure after treatment with an oral artemisinin-based monotherapy with adequate antimalarial blood concentration, as evidenced by the persistence of parasites for 7 days, or the presence of parasites at day 3 and recrudescence within 28/42 days (confirmed resistance) [10]. Because of the influence of external factors (host immunity, variations of drug absorption and metabolism), the results of in vivo tests do not necessarily reflect the true level of pure antimalarial resistance but provides the best information on the efficacy of antimalarial treatment [11].
Keeping these facts in mind, this study aimed to detect the prevalence of clinical Artemisinin resistance among complicated malaria cases in this region, by invivo technique, according to WHO 1996 manual [12] and WHO 2011 update on detection of artemsinin resistance [10].
This study will help in detecting prevalence of artemisinin resistance in our region and will aid the treating physician for more selective use of drug-combinations which are less likely to foster resistance, ensure patient compliance for drug intake and implement follow-up practices to detect cases of drug-resistance.
Materials and Methods
The present study was conducted prospectively at the Department of Microbiology, Maharaja Krushna Chandra Gajapati (MKCG) Medical College and Hospital, Berhampur, India, for a period of two years from September 2013 to August 2015, including period of follow-up, with active collaboration of Departments of Medicine, Paediatrics and Obstetrics and Gynaecology. The study group comprised of 563 clinically suspected cases of complicated malaria admitted to in-patient Departments of Medicine, Paediatrics and Obstetrics and Gynaecology, MKCG Medical College and Hospital, Berhampur, Odisha, India. This study was approved by the Institutional Ethical Committee (ECR/661/Inst/OR/2014/RR-17). Oral and informed consent to participate in the study was obtained from the adult patients and guardians of young children.
Inclusion criteria: Clinically suspected cases of complicated malaria with complains of fever and features of complication like altered sensorium, convulsions, renal impairment, jaundice, respiratory distress, severe anaemia, bleeding manifestations were included in the study group.
Exclusion criteria: Patients with fever but without any features of complicated malaria as mentioned above and those patients with confirmed diagnosis of infections like typhoid, pneumonia, urinary tract infection, septicaemia, meningitis, encephalitis, gastrointestinal infections, dengue, or leptospirosis were ruled out from the study.
Collection of data: Patient’s details including age, sex, address, presenting signs and symptoms, any history of antimalarial treatment taken in previous four weeks and provisional clinical diagnosis were noted.
Collection of specimen: About five mL of EDTA anticoagulated venous blood was collected with all aseptic precautions and brought to the laboratory. It was subjected to thick and thin smear (Giemsa stain) examination immediately, according to the WHO manual [13].
Blood was also sent to haematology and biochemistry laboratory for evaluation of other parameters of complicated malaria. Test done were: Estimation of haemoglobin by Sahli’s haemoglobinometer, total RBC count by Neubaur’s chamber, Total Platelet Count by Neubaur’s chamber, fasting blood sugar estimation in automated analyser, total bilirubin estimation, serum creatinine, liver enzymes Aspartate aminotransferase (AST or SGOT) and Alanine Aminotransferase (ALT or SGPT). Results of all tests were recorded.
Detection of clinical artemsinin resistance: Invivo method [10,12]: According to the modified WHO antimalarial drug-resistance protocol, each patient was followed for period of 28 days. Patients were assessed on Day 0, Day 1, Day 2, Day 3, Day 7, Day 14 and Day 28. Clinical assessment for recovery and axillary temperature were recorded on Day 0, Day 1, Day 2, Day 3, Day 7, Day 14 and Day 28. Parasitological examination was done Day 0, Day 1, Day 3, Day 7, Day 14 and Day 28 for all diagnosed complicated malaria cases.
On Day 0 (On the day of presentation): Patient’s clinical assessment for presence of danger signs (altered sensorium, convulsions, vomiting, prostration) and body temperature were recorded. Microscopic blood film examination and quantification of parasitaemia level by thick smear were done before administration of Artesunate therapy. The details were recorded. Patients were then started on intravenous Artesunate monotherapy (2.4 mg/kg body weight).
On Day 1: Patients were assessed clinically for presence of any danger signs. Axillary temperature was recorded. Finger-prick blood was collected and Giemsa stained thick smears were done to look for presence of parasites. If asexual forms of parasites were detected, quantification of parasitaemia was done.
On Day 2: Patients were assessed clinically for presence of any danger signs (altered sensorium, convulsions, vomiting, prostration). Axillary temperature was recorded.
On Day 3, Day 7, Day 14 and Day 28: Patients were assessed clinically for presence of any danger signs (altered sensorium, convulsions, vomiting, prostration). Axillary temperature was recorded. Finger-prick blood was collected and Giemsa stained thick smears were done to look for presence of parasites. If asexual forms of parasites were detected, quantification of parasitaemia was done.
Interpretation of results of invivo follow-up parasitological examination after Artesunate monotherapy [10,12]:
Result on Day 1: If clinical recovery was seen and axillary temperature was ≤37.5°C, along with parasitological examination showing marked reduction or no parasites after 24 hours of therapy, patients were considered to be responding to Artesunate monotherapy. If clinical recovery was not evident and axillary temperature was ≥37.5°C and parasitaemia levels remained same or increased, patients were designated as ‘not responding’ to therapy. But Artesunate was continued and patients were followed-up on Day 2 for clinical assessment.
Result on Day 2: If clinical recovery was seen and axillary temperature was ≤37.5°C, then patients were considered to be responding well to Artesunate monotherapy. If clinical recovery was not evident and axillary temperature was ≥37.5°C, then Artesunate was continued and patients were followed-up on Day 3 for parasitological examination.
Result on Day 3: If clinical recovery was seen and axillary temperature was ≤37.5°C, and no parasites were detected on follow-up parasitological examination, then patients were designated as ‘Sensitive’ to Artesunate monotherapy or might be resistant to therapy at RI level. If clinical recovery was not evident and axillary temperature was ≥37.5°C, and asexual forms of parasites were detected on thick smear examination, then patients were suspected to be resistant to Artesunate monotherapy. Quantification of parasitaemia was done. If ≥25% asexual forms of parasites persisted, the patients were suspected to be resistant to Artesunate monotherapy at RII level. If ≥75% asexual forms of parasites persisted, the patients were suspected to be resistant to Artesunate monotherapy at RIII level. The treating clinician was informed of suspected drug resistance to Artesunate monotherapy.
Result on Day 5: Clinical assessment and parasitological examination was done only in cases of suspected Artesunate resistance cases that were detected on Day 3.
Result on Day 7: If clinical recovery was seen and axillary temperature was ≤37.5°C, and no parasites were detected on follow-up thick smear parasitological examination, then patient was designated as ‘Sensitive’ to Artesunate monotherapy or might be resistant to therapy at RI level. If clinical symptoms reappeared and axillary temperature was ≥37.5°C, and asexual forms of parasites were detected on thick smear examination, then patients were designated as ‘Resistant’ to Artesunate monotherapy. Quantification of parasitaemia was done. If ≥25% asexual forms of parasites persisted, the patients were designated as resistant to Artesunate monotherapy at RII level. If ≥75% asexual forms of parasites persisted, the patients were designated as resistant to Artesunate monotherapy at RIII level.
Result on Day 14: If clinical recovery was seen and axillary temperature was ≤37.5°C, and no parasites were detected on follow-up thick smear parasitological examination, then patients were designated as ‘Sensitive’ to Artesunate monotherapy or might be resistant to therapy at RI level. If patients showed clinical symptoms again and parasites were detected on follow-up thick smear parasitological examination, then patients were designated as ‘Resistant’ to Artesunate monotherapy at RI level.
Result on Day 28: If patients had completely recovered clinically and no recrudescent parasites were found on follow-up thick smear parasitolgical examination, then patients were labelled as ‘Sensitive’ to Artesunate monotherapy. But if fever and any other associated clinical features of malaria reappeared and parasitological examination showed recrudescent asexual forms of parasites, then patients were labelled as ‘Resistant’ to Artesunate monotherapy at RI level.
Results
The following findings were observed and analysed in present study. Out of total number of 563 clinically diagnosed malaria cases, 355 (63.1%) were male and 208 (36.9%) were females. The predominant numbers of clinically diagnosed complicated malaria were in the age group 15-45 years in both the sexes [Table/Fig-1]. Fever (100%) was present in all cases. The most common associated complication was anaemia (40.5%) followed by jaundice (32.5%), [Table/Fig-2].
Age and sex distribution of the study group (n=563).
| Age groups (years) | Cases |
|---|
| Male n (%) | Female n (%) |
|---|
| 0-14 | 45 (8.0%) | 28 (5.0%) |
| 15-25 | 82 (14.6%) | 44 (7.8%) |
| 26-35 | 122 (21.7%) | 75 (13.3%) |
| 36-45 | 57 (10.1%) | 39 (6.9%) |
| 46-55 | 27 (4.8%) | 17 (3%) |
| ≥56 | 22 (3.9%) | 5 (0.9%) |
| Total | 355 (63.1%) | 208 (36.9%) |
Clinical profile of complicated malaria in the study group (n=563).
| Clinical presentation | No. of cases |
|---|
| Fever | 563 (100%) |
| Anaemia | 228 (40.5%) |
| Jaundice | 183 (32.5%) |
| Altered sensorium | 91 (16.1%) |
| Convulsions | 13 (13.8%) |
| Vomiting | 46 (5.2%) |
| Bleeding manifestation | 09 (1.5%) |
Out of 563 cases of clinically diagnosed complicated malaria, 241 (42.8%) were found to be positive for malaria parasite. Among the positive cases, 204 cases showed trophozoites of Plasmodium falciparum, 32 cases showed Plasmodium vivax trophozoites and five cases of mixed infection showed both types of trophozoites [Table/Fig-3]. Most of the cases, 87(36.1%) had severe anaemia, with Hb<5 g/dL, while raised bilirubin was seen in 78 (32.4%) cases [Table/Fig-4]. Clinical recovery was indicated, when patient’s body temperature was ≤37.5°C and patient did not show any evidence of danger signs (vomiting, altered sensorium, convulsions). All the 32 cases of Plasmodium vivax and five cases of mixed infection, clinically responded to treatment with artesunate monotherapy and they recovered. Out of 204 cases of Plasmodium falciparum, 198 cases responded to artesunate monotherapy and clinical recovery was observed. But fever and other clinical signs persisted in 6 cases. Clinical resistance to artesunate monotherapy was suspected in these cases [Table/Fig-5].
Detection of malaria positive cases by thick smear examination and differentiation of Plasmodium species by thin smear examination (n=563).
| Clinically diagnosed cases (n=563) |
|---|
| Malaria positive cases | 241 (42.8%) | Plasmodium falciparum | Plasmodium vivax | Mixed infection |
| 204 | 32 | 5 |
| Negative for malaria | 322 (57.2%) |
Pathological and biochemical investigations in the complicated malaria cases (n=241).
| Laboratory finding | No. of cases (n=241) |
|---|
| Hb <5 g/dL | 87 (36.1%) |
| Thrombocytopenia | 4 (1.7%) |
| Serum bilirubin >2.5 g/dL | 78 (32.4%) |
| ALT raised | 40 (16.6%) |
| SGOT/SGPT | 64 (26.6%) |
| Serum creatinine >3 g/dL | 7 (2.9%) |
| FBS <40 mg% | 16 (6.6%) |
Hb: Haemoglobin; ALT: Alanine transaminase; AST: Aspartate aminotransferase; SGOT: Serum glutamic oxaloacetic transaminase; SGPT: Serum glutamic pyruvic transaminase; FBS: Fasting blood sugar; AST: Aspartate aminotransferase
Clinical response of cases to artesunate monotherapy (n=241).
| Species | No. of cases | **Clinical recovery by Day 5 | Deterioration/No recovery by Day 5 |
|---|
| P. falciparum | 204 | 198 | 6 |
| P. vivax | 32 | 32 | - |
| Mixed infection (Pf and Pv) | 5 | 5 | - |
| Total | 241 | 235 | 6 |
Only thick smear positive cases were followed-up for detection of drug resistance.
**Clinical recovery was indicated, when patient’s body temperature was ≤37.5°C and patient did not show any evidence of danger signs (vomiting, altered sensorium, convulsions)
All the 241 cases of complicated malaria that were diagnosed in the laboratory had a parasitaemia level varying from 5000-10000 on day 0, before administration of artesunate therapy. In 235 patients (which included all 32 cases of Plasmodium vivax, all five cases of mixed infection and 198 out of 204 cases of Plasmodium falciparum), there was complete clearance of parasitaemia on day 3 after artesunate monotherapy. On follow-up parasitological examination, no asexual parasites were detected in these cases on days 7 and 14. In the remaining six cases of Plasmodium falciparum malaria, parasitaemia persisted even after 3rd day and continued to persist till fifth day of artesunate monotherapy. One patient succumbed to death on the 4th day of therapy. An additional parasitological examination was done on the 5th day in the suspected resistant cases before change of therapy. The details of these are given in [Table/Fig-6].
Details of clinical artesunate resistant cases (in vivo method).
| Drug-resistant cases | Level of parasitaemia on thick smear examination |
|---|
| Day 0 | Day 1 | Day 3 | Day 5 | Day 7 | Day 14 | Day 28 | Degree of resistance |
|---|
| Case 1 | 8600 | 3200 | 1120 | 1080 | Nil | Nil | Nil | R II |
| Case 2 | 7680 | 4280 | 1240 | 880 | Nil | Nil | Nil | R II |
| Case 3 | 7520 | 3320 | 1160 | 1040 | Nil | Nil | Nil | R II |
| Case 4 | 8200 | 4000 | 1360 | 1240 | Nil | Nil | Nil | R II |
| Case 5 | 8800 | 7640 | 6720 | 6640 | Nil | Nil | Nil | R III |
| Case 6 | 9680 | 9880 | 11200 | Death occurred on day 4 | - | - | - | R III |
R II- Parasitaemia reduced to 25% or less than pre-treatment levels after first 48 hours of therapy. R III- Parasitaemia reduced to less than 75% of pre-treatment level or remain at the same level or increase in level after first 48 hrs of therapy
Level of Parasitaemia on Thick Smear Examination [
Table/Fig-7]
Levels of parasitaemia seen among the six cases of clinical artesunate resistance on the day before administration of therapy and post therapy days.
*Case 6 expired on day 4; RII- Parasitaemia reduced to 25% or less than pre-treatment levels after first 48 hours of therapy. RIII- Parasitaemia reduced to less than 75% of pre-treatment level or remain at the same level or increase in level after first 48 hours of therapy
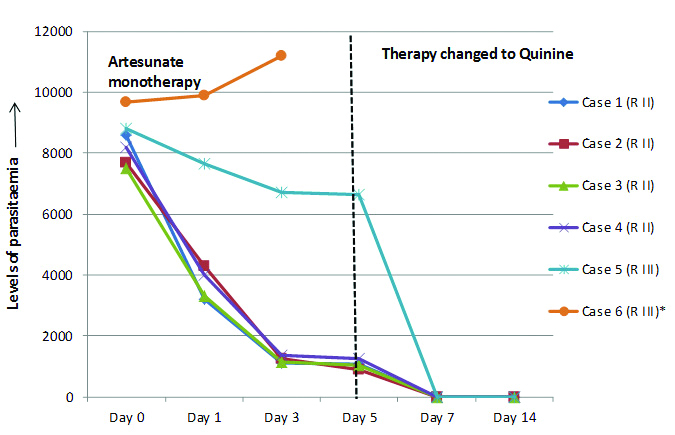
Day 0- Case was detected as malaria in the laboratory with quantification of parasitaemia followed by commencement of intravenous artesunate monotherapy.
Day 1- Quantification of parasitemia was done after 24 hours of therapy. Parasite levels were reduced in case no. 1, 2, 3 and 4. But levels remained slightly reduced in case 5. In case 6, parasite level was increased, as compared to Day 0.
Day 3- In case no. 1, 2, 3 and 4, less than 25% parasitaemia persisted after 3rd day of treatment. In case no. 5 and 6, more than 75% parasites persisted after 3rd day of therapy. Artesunate monotherapy was continued in all 6 cases upto 5 days to complete course of treatment.
Day 5- In case no. 1, 2, 3 and 4, less than 25% parasitaemia persisted even after fifth day of treatment. Fever reappeared in these cases. Thus, parasite was designated as ‘resistant’ at RII level.
In case no. 5, more than 75% parasites persisted without remission of fever, and also showed evidence of danger signs despite adequate artesunate monotherapy. Here, parasite was said to be ‘resistant’ at RIII level. Therapy was changed to intravenous Quinine in all these cases. But in case 6, the patient succumbed to the disease on day 4 due to complications.
Day 7- Case no. 1, 2, 3, 4 and 5 (where therapy with artesunate was replaced by quinine) showed marked clinical improvement and no asexual forms of parasites was detected on day 7.
Day 14- No asexual forms of parasites were detected in the five cases.
Out of 241 cases, 235 cases were sensitive to artesunate monotherapy. RII level of resistance was detected in 1.96% cases while RIII level of resistance was detected in 0.98% cases. RI resistance was not detected in any case [Table/Fig-8]. Prevalence of clinical artesunate resistance was detected in 2.94% of cases.
Degree of clinical artesunate resistance to among Plasmodium falciparum cases.
| Response | No. of cases (n=204) | Percentage |
|---|
| Sensitive | 198 | 97.06% |
| RI | - | - |
| RII | 4 | 1.96% |
| RIII | 2 | 0.98% |
Sensitive- No asexual/sexual forms found on day 7. No reappearance of asexual forms by follow-up till day 14. RI- Clearance of asexual parasitaemia for 2 consecutive days, and reappearance of ring forms on day 7. RII- Parasitaemia reduced to 25% or less than pre-treatment levels after first 48 hours of therapy. RIII- Parasitaemia reduced to less than 75% of pre-treatment level or remain at the same level or increase in level after first 48 hours of therapy
Discussion
Once the complicated malaria cases were confirmed in the laboratory for presence of malarial parasite, they were then administered intravenous artesunate monotherapy. Following therapy, clinical assessment and parasitological examination was done to observe the response of patients to antimalarial therapy according to WHO 1996 protocol for detection of therapeutic efficacy in antimalarials and the WHO 2011 update on detection of artemisinin resistance [10,12]. In the present study, a total of 563 patients were included, among which only 241 cases were positive for presence of malarial parasites. Out of the 241 malaria positive cases, 204 cases were due to Plasmodium falciparum, 32 cases were due to Plasmodium vivax and five cases were of mixed infection.
After administration of artesunate monotherapy, clinical recovery was evident on fifth day of therapy in all 32 cases of Plasmodium vivax, all five cases of mixed infection and 198 cases of P. falciparum. But 6 (2.94%) of P. falciparum cases did not show clinical recovery by fifth day of therapy. Clinical resistance to artesunate monotherapy was suspected in these cases. Therefore, therapy was changed to quinine, in the suspected resistant cases, following a parasitological examination on the fifth day. Similar cases of resistance to artesunate and delayed parasite clearance have been reported in the WHO update from Myanmar, Thailand and Cambodia [14]. Rogers WO et al., have also reported failure of artesunate therapy from southern Cambodia [14]. A study from North-Eastern India also reveals treatment failure rates following artesunate therapy [15]. Results of the present study were comparable to these studies. A case of resistance to artesunate monotherapy has also been reported from Kolkata, India [16].
In the present study, all 241 cases of complicated malaria, that were diagnosed in the laboratory, had a parasitaemia level varying from 5000-10000 on day 0, before administration of artesunate therapy. In 235 patients (which included 32 cases of P.vivax, five cases of mixed Plasmodium falciparum and Plasmodium vivax infection and 198 cases of Plasmodium falciparum), complete clearance of parasitemia was seen on 3rd day of artesunate monotherapy. In the remaining six cases of Plasmodium falciparum complicated malaria, parasitaemia persisted after 3rd day of artesunate monotherapy. In four (1.96%) cases, less than 25% parasitaemia persisted, which were designated as ‘resistant’ at R II level, whereas more than 75% parasitaemia persisted in remaining two (0.98%) cases, designating these cases to be ‘resistant’ at R III level. This may be due to delayed clearance of parasites by artesunate monotherapy as is revealed by the WHO status update on artemisinin resistance [15]. Similar results are reported by the study of Mishra N et al., and the study of case report of artemisinin from Kolkata by Bhattacharya N et al., [15,16].
Prevalence of artesunate resistance in this region was found to be 2.94% among the Plasmodium falciparum cases, in the present study by invivo method. Due to certain limitations, the molecular study for detection of artemisinin resistance gene could not be performed. The PfATPase6, which is the product of the pfserca gene, has been suggested to be a specific target of the artemisinin drugs [17]. The point mutation and copy number variation of multi-drug resistance gene1 (pfmdr1), mutation of the gene encoding sarco-endoplasmic reticulum calcium ATPase6(pfserca), mitochondria genome (mt DNA) and deubiquitinating enzyme (pfubp-1) showed a significant correlation with artemisinin resistance in the studies conducted in South-East Asia [18]. Mutations that change the primary amino-acid sequence of the so-called propeller region of the kelch motif containing gene, known as K13, have been identified as a key causal determinant of artemisinin resistance [6]. Mutations in the Kelch 13 (K13)-propeller domain were shown to be associated with delayed parasite clearance in vitro and in vivo. Analysis of the recently identified molecular marker for artemisinin resistance showed that the C580Y mutation was the most prevalent in parts the Greater Mekong subregion, but a total of 186 K13 alleles mutations, in and near the K13 propeller region were also found to be associated with artemisinin resistance (e.g., Y493H, R539T, I543T) [19]. In vitro resistance to artemether in parasites obtained from French Guiana (but not Cambodia) was linked to the S769N mutation in pfserca. In the Greater Mekong Sub region, Thailand and Cambodia, high treatment failure rate following treatment with an ACT has only been observed where resistance to the partner drug exists, regardless of the presence artemisinin resistance [14].
Limitation(s)
The present study could only detect cases of partial artemisinin resistance. The confirmation of the suspected cases of clinical artemisinin resistance could have been done by detecting the molecular marker of artesunate resistance, the mutations in the Kelch-13 propeller domain [19]. Since, the reliability of the molecular marker was not known; the confirmation of resistance could not be done.
Conclusion(s)
Follow-up of all cases should be done by thick smear examination to detect the response of the cases towards the administered antimalarial drugs. In the present study, resistance to artesunate monotherapy was detected in 2.94% of complicated Plasmodium falciparum cases, by invivo method. Hence, Artemisinin-based Combination Therapies should be used as the first-line antimalarial in all complicated malaria cases to combat the rapidly emerging artesunate resistance. Global screening for resistance will help to estimate the magnitude of the problem and to search for an alternative antimalarial drug.
Hb: Haemoglobin; ALT: Alanine transaminase; AST: Aspartate aminotransferase; SGOT: Serum glutamic oxaloacetic transaminase; SGPT: Serum glutamic pyruvic transaminase; FBS: Fasting blood sugar; AST: Aspartate aminotransferase
Only thick smear positive cases were followed-up for detection of drug resistance.
**Clinical recovery was indicated, when patient’s body temperature was ≤37.5°C and patient did not show any evidence of danger signs (vomiting, altered sensorium, convulsions)
R II- Parasitaemia reduced to 25% or less than pre-treatment levels after first 48 hours of therapy. R III- Parasitaemia reduced to less than 75% of pre-treatment level or remain at the same level or increase in level after first 48 hrs of therapy