Necrosis of Anterior Wall of Stomach Presenting as Pneumoperitoneum in Neonates- Report of Two Cases with Review of Literature
Subrat Kumar Mohanty1, Harish Chandra Tudu2, Amaresh Mishra3, Pran Singh Pujari4, Nemani Prashanthi5
1 Professor, Department of Surgery and Consultant Paediatric Surgeon, KIMS, Bhubaneswar, Odisha, India.
2 Consultant Paediatric Surgeon, Department of Paediatric Surgery, KIMS, Bhubaneswar, Odisha, India.
3 Professor, Department of General Surgery, KIMS, Bhubaneswar, Odisha, India.
4 Professor and Head, Department of General Surgery, KIMS, Bhubaneswar, Odisha, India.
5 Postgraduate, Department of General Surgery, KIMS, Bhubaneswar, Odisha, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Subrat Kumar Mohanty, Professor, Department of Surgery and Consultant Paediatric Surgeon, KIMS, Bhubaneswar-751024, Odisha, India.
E-mail: subrat.mohanty@kims.ac.in
Necrotising enterocolitis is a common disease that leads to low birth weight and preterm babies. Mostly small intestine and large intestine develop necrosis sparing stomach and duodenum. Necrosis involving the anterior wall of stomach and sparing other parts of Gastrointestinal (GI) tract is a rare entity, but can present in neonates with massive pneumoperitoneum. Though the exact aetiology is not known, it may be related to perinatal hypoxia. Case report of two preterm low birth weight babies who presented with pneumoperitoneum on day three of life had an area of anterior gastric wall necrosis with perforation peritonitis. The rest of GI tract was healthy. After excision of necrotic tissue, suture repair of viable stomach and feeding jejunostomy was done in both cases. These two neonates survived with intensive neonatal ICU care and gradual jejunostomy feeding. Gastric wall necrosis with perforation peritonitis is a rare entity in neonates with high mortality. Tip of nasogastric tube lying outside stomach shadow can give clue to the diagnosis. Survival can be improved by early surgery, good neonatal Intensive Care Unit (ICU) care and gradual jejunostomy feeding.
Gastric necrosis, Neonatal, Perforation
Case 1
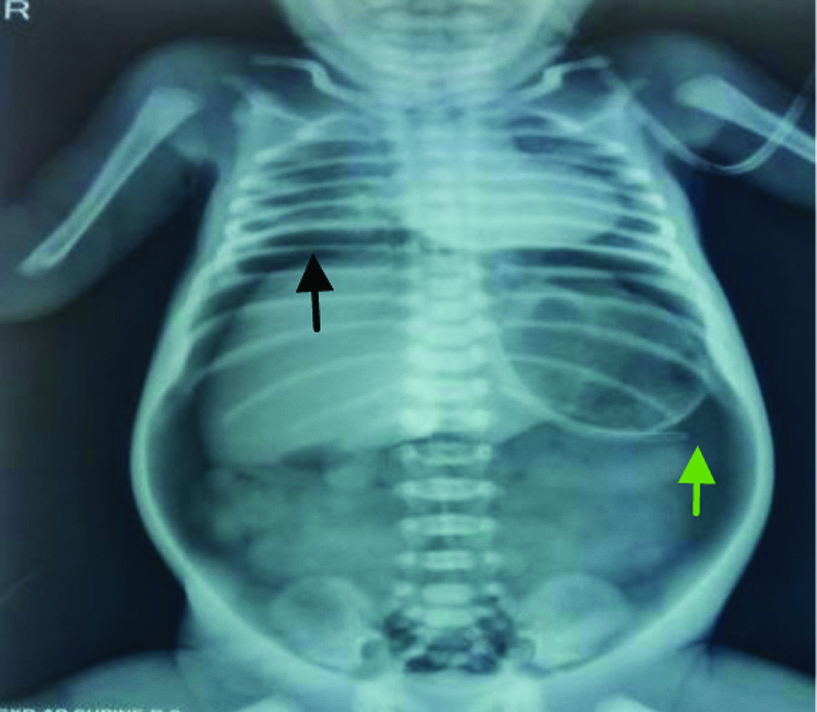
A 32-week-old premature male baby of weight 1500 gm after lower Caesarean section delivery presented to our neonatal emergency with mild respiratory distress on day one of life and was kept in neonatal ICU for observation. Subsequently, he needed intubation and ventilator support. On day two, the child developed abdominal distension and still required ventilator support. Repeat X-ray abdomen on day three showed gas under diaphragm with tip of the nasogastric tube lying outside the gastric shadow [Table/Fig-1].
Gas under diaphragm (Black arrow) with nasogastric tube outside gastric shadow (Green arrow).
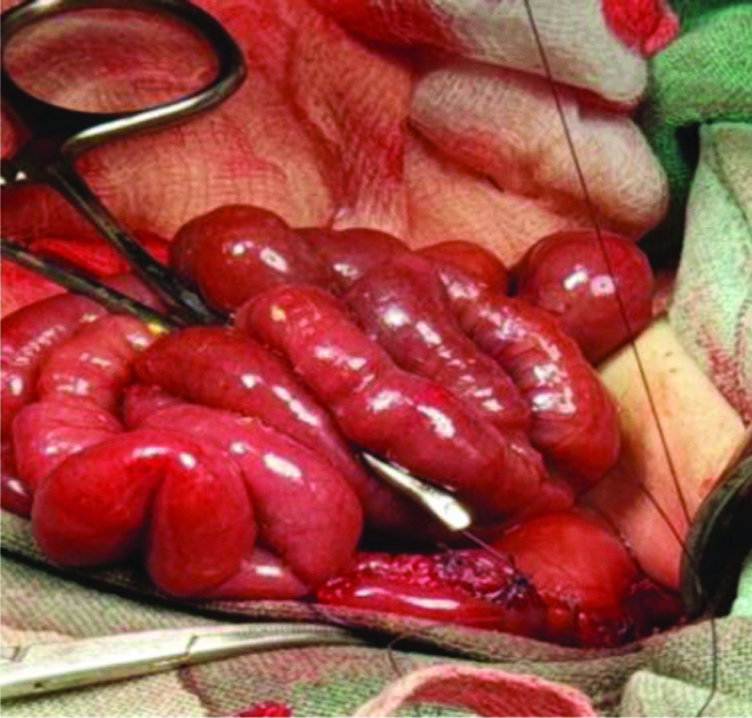
Exploratory laparotomy showed massive necrosis of anterior gastric wall extending from the lesser curvature to greater curvature of stomach with perforation. The necrotic tissue including perforation was excised, viable tissue was suture repaired (gastrorrhaphy) after trimming the margins [Table/Fig-2] with feeding jejunostomy.
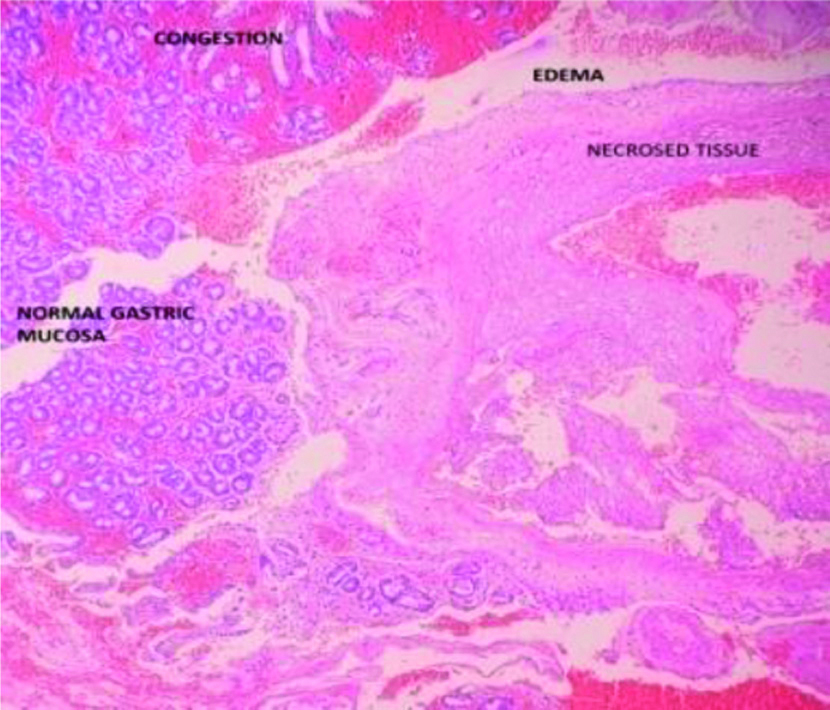
Biopsy of the margins showed areas of necrosis and haemorrhage in mucosa [Table/Fig-3].
Biopsy showing necrosis {Haematoxylin-Eosin (H&E) staining, magnification 100x}.
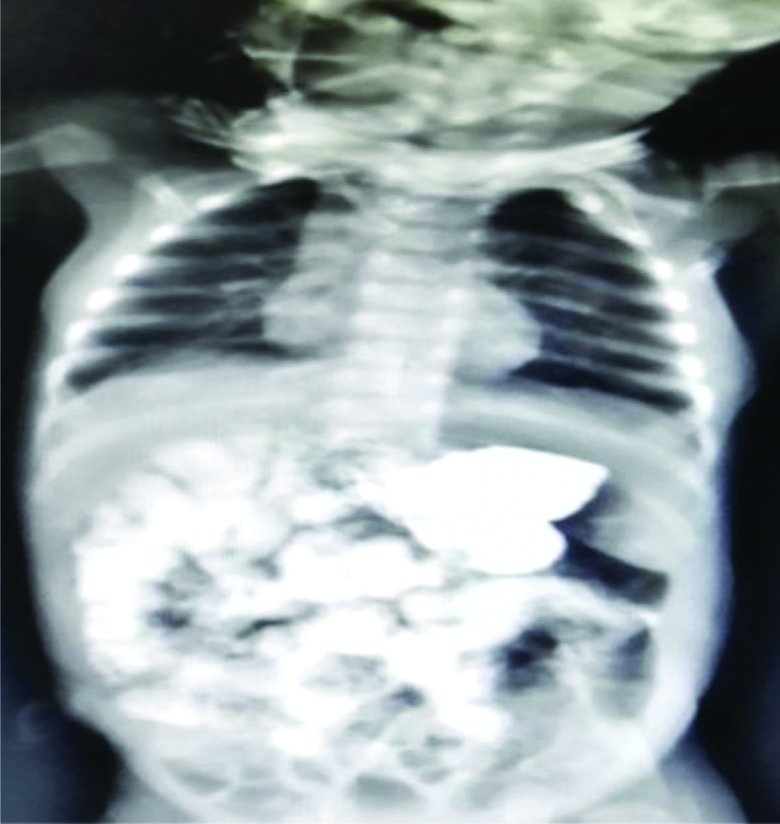
Patient had a smooth recovery in neonatal ICU; jejunostomy feeding was started on postoperative day five and gradually increased to full feeding. After three weeks upper GI contrast study showed no leak with a small capacity stomach. Patient was put on gradual increased oral feeding and gained weight up to 2.5 kg before the feeding jejunostomy removed at the end of two months. Barium dye study after two months showed mild increase in the capacity of the stomach with child tolerating full oral feeding [Table/Fig-4]. He is currently eight-month-old and weighs 6.3 kg.
Barium dye study after two months.
Case 2
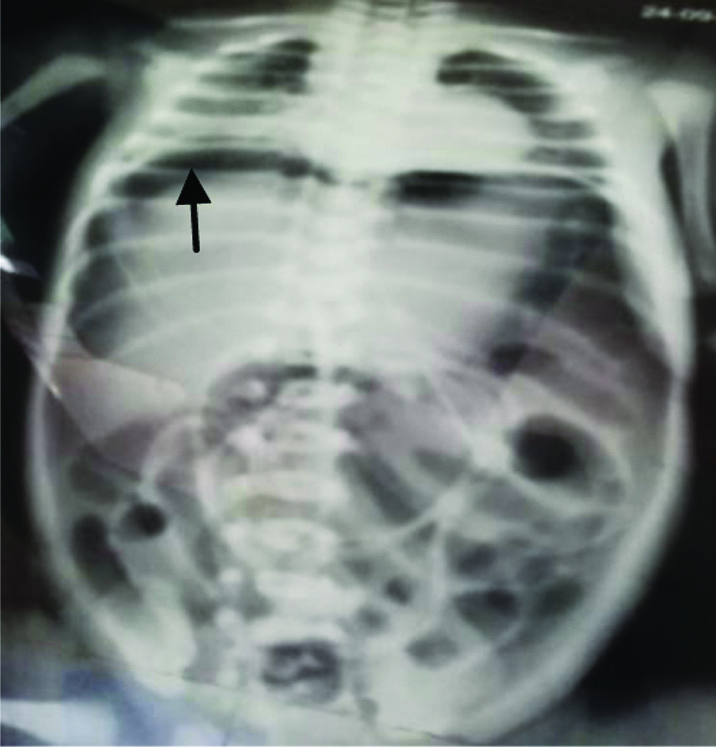
First preterm female baby of 32 weeks of twin pregnancy with normal vaginal delivery of weight 1400 grams was referred to our emergency on day three of life due to abdominal distension after oral feeding. X-ray abdomen showed massive pneumoperitoneum and patient was in mild respiratory distress [Table/Fig-5].
Pneumoperitoneum (Black arrow) in second case.
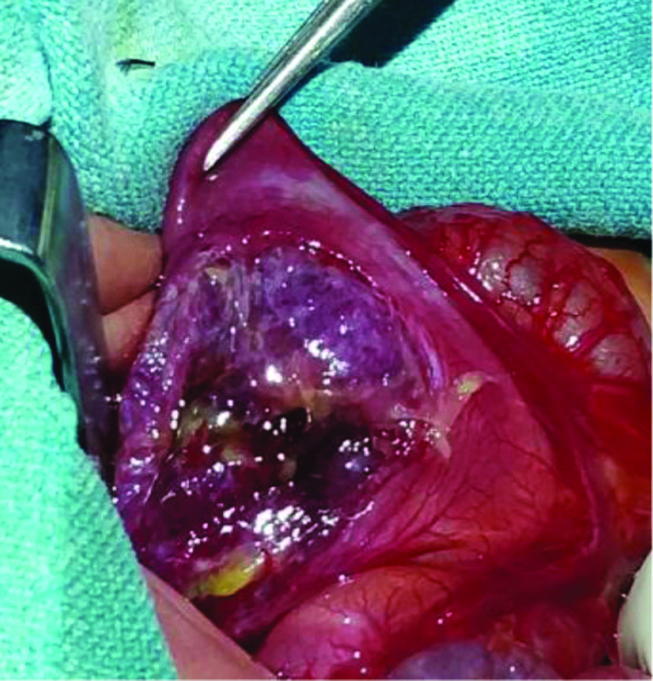
Arterial blood analysis was within normal limit with total leucocyte count 12000/mm3 and CRP 15 mg/L. On admission to ICU, neonate was managed with only oxygen hood. Bilateral flank drain was given immediately due to severe abdominal distention. Laparotomy done after the child was stabilised showed necrosis of anterior wall of stomach [Table/Fig-6], which was excised and repaired in two layers with a feeding jejunostomy.
Necrosis of anterior wall of stomach.
Some of the other causes of pneumoperitoneum like spontaneous perforation of hollow viscus, necrotising enterocolitis and intestinal obstruction like hirschsprung’s disease were considered preoperatively.
Patient recovered well in neonatal ICU with jejunostomy feeding and was discharged after three weeks following upper GI barium study and gradual increase of oral feeding [Table/Fig-7].
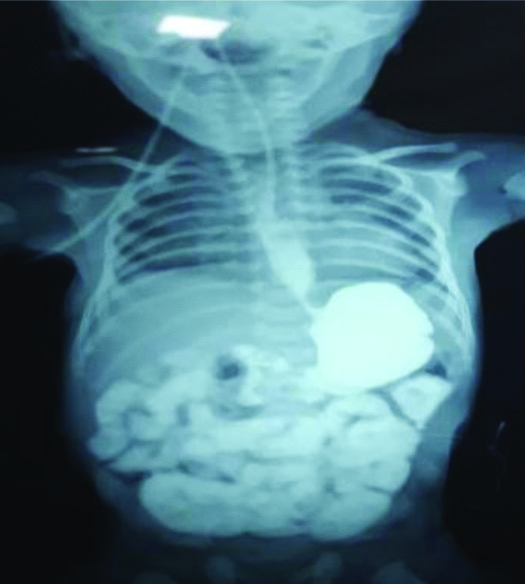
Barium dye study after three weeks (second case).
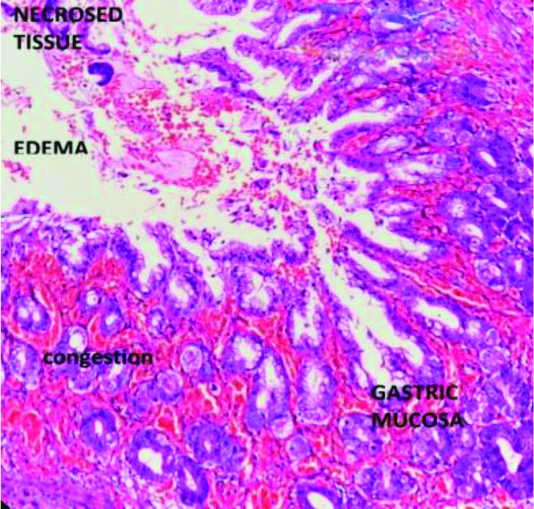
Feeding jejunostomy removed after six weeks. Biopsy showed area of necrosis with nonspecific inflammation [Table/Fig-8]. Patient is on follow-up with weight 4.2 kg and doing well after six months.
Biopsy showing necrosis with inflammation, {Haematoxylin-Eosin (H&E) staining, magnification 10x}.
Informed consent was obtained from the guardians of the subjects involved in the study.
Discussion
Neonatal isolated gastric necrosis and perforation is a rare life-threatening entity. Spontaneous gastric perforation is more often seen in children than adults in intensive care unit [1]. Incidence is 1:5000 live births and accounts for 7% of all gastrointestinal perforations in newborns [2]. Around 200 cases have been documented in the last decade as per literature [3]. Aetiology of this condition is unclear. However hypoxia, respiratory distress, and sepsis associated with prematurity (before thirty seven completed weeks of gestation) and low birth weight (less than 2499 gm) can predispose to ischemia of stomach [4]. In a literature review by Lin C et al., incidence of neonatal gastric perforation was 52% in low birth weight babies [5]. This is due to immaturity of the gastric tissue and also lack of intestinal pacemaker cells, and lack of C-KIT mast cell, thereby affecting the coordination of gastric motility leading to hypomotility and perforation [6]. In a study, of 66 case series and literature review by Yang T et al., 50 perforations occurred in the greater curvature of the stomach (73.5%), 12 in the lesser curvature (17.6%), and 6 were unspecified [7]. Necrosis of anterior gastric wall is less common and was observed in our both cases. In a systemic review by Chen TY et al., mortality rate was not significant in different location of perforation [8]. In a premature neonate intrinsic fragile gastric wall more often give way to perforation even in a trivial traumatic episode like putting nasogastric tube or bag mask ventilation. Circulatory regulations are not fully developed leading to poor circulation, tissue hypoxia with necrosis and sepsis [9]. Anatomical anomalies like gastric outlet obstruction, duodenal web may predispose to neonatal gastric perforation in both term and preterm neonates. It is also associated with postnatal steroid therapy [10].
Clinical features are acute abdominal distention, feeding intolerance, lethargy, GI bleeding, respiratory distress and sepsis [11]. Sepsis is most significantly associated with mortality. Peak incidence of spontaneous gastric perforation is in the first week between two to seven days of life [12].
X-ray is the earliest detecting modality documenting pneumoperitoneum. Ultrasound guided peritoneal drainage can be done in unstable patients, but surgery is the definitive modality of the treatment as followed in second case. Debridements of the necrotic tissue with gastrorraphy are commonly performed procedure alone or combined with gastrostomy [7,9]. In both cases debridement of the necrotic tissue with gastrorraphy was done along with feeding jejunostomy. Feeding jejunostomy was done in both our patients as safety measure, giving rest to the sutured gastric wall, initiate early enteral feeding and minimise infection by bacterial translocation in the gut over total parenteral nutrition.
Poor prognostic factors are male sex, prematurity, hyponatremia (Serum. Sodium <130 mmol/L) and metabolic acidosis (pH <7.3) [13]. Mortality is very high due to sepsis with respiratory failure and associated prematurity. But over the years mortality has decreased mainly due to the considerable improvements in the intensive care for critically ill neonates [14].
Conclusion(s)
Isolated neonatal gastric necrosis with perforation is very rare and aetiology of this condition is poorly understood and related to neonatal stress. Preterm and low birth weight is the two main contributing factors in more than 50% of the cases. Prompt diagnosis and definitive surgical management at the earliest can reduce the mortality. On X-ray abdomen, tip of the nasogastric tube lying free inside peritoneal cavity may be an important finding in few cases. In some selected cases feeding jejunostomy is very useful both in early enteral feeding and also giving rest to the sutured stomach.
Author Declaration:
Financial or Other Competing Interests: None
Was informed consent obtained from the subjects involved in the study? Yes
For any images presented appropriate consent has been obtained from the subjects. Yes
Plagiarism Checking Methods: [Jain H et al.]
Plagiarism X-checker: May 13, 2020
Manual Googling: Jul 18, 2020
iThenticate Software: Aug 22, 2020 (10%)
[1]. Grosfeld JL, Molinari F, Chaet M, Engum SA, West KW, Rescorla FJ, Gastrointestinal perforation and peritonitis in infants and children: Experience with 179 cases over 10 yearsSurgery 1996 120(4):650-56.10.1016/S0039-6060(96)80012-2 [Google Scholar] [CrossRef]
[2]. Aydin M, Deveci U, Taskin E, Bakal U, Kilic M, Percutaneous peritoneal drainage in isolated neonatal gastric perforationWorld J Gastroenterol 2015 21:12987-88.10.3748/wjg.v21.i45.1298726668521 [Google Scholar] [CrossRef] [PubMed]
[3]. Gathwala G, Rattan KN, Abrol P, Sodhi D, Spontaneous Gastric perforation in a neonateIndian Pediatr 1994 31:1013-15. [Google Scholar]
[4]. Terui K, Iwai J, Yamada S, Takenouchi A, Nakata M, Komatsu S, Etiology of neonatal gastric perforation: A review of 20 years’ experiencePediatr Surg Int 2012 28:09-14.10.1007/s00383-011-3003-422009207 [Google Scholar] [CrossRef] [PubMed]
[5]. Lin CM, Lee HC, Kao HA, Hung HY, Hsu CH, Yeung CY, Neonatal gastric perforation: Report of 15 cases and review of the literaturePediatr Neonatol 2008 49:65-70.10.1016/S1875-9572(08)60015-7 [Google Scholar] [CrossRef]
[6]. Durham MM, Ricketts RR, Neonatal gastric perforation and necrosis with Hunt-Lawrence pouch reconstructionJ Pediatr Surg 1999 34:649-51.10.1016/S0022-3468(99)90097-0 [Google Scholar] [CrossRef]
[7]. Yang T, Huang Y, Li J, Zhong W, Tan T, Yu J, Neonatal gastric perforation: Case series and literature reviewWorld J Surg 2018 42(8):2668-73.10.1007/s00268-018-4509-x29392435 [Google Scholar] [CrossRef] [PubMed]
[8]. Chen TY, Liu HK, Yang MC, Yang YN, Ko PJ, Su YT, Neonatal gastric perforation: A report of two cases and a systemic reviewMedicine (Baltimore) 2018 97(17):e036910.1097/MD.000000000001036929702982 [Google Scholar] [CrossRef] [PubMed]
[9]. Iacusso C, Boscarelli A, Fusaro F, Bagolan P, Morini F, Pathogenetic and prognostic factors for neonatal gastric perforation: Personal experience and systematic review of the literatureFrontiers. Pediatr 2018 6:6110.3389/fped.2018.0006129670869 [Google Scholar] [CrossRef] [PubMed]
[10]. Behrman RE, Kliegman RM, Jenson HB, Nelson textbook of pediatrics. In: Stoll BJ, Kleigman RM, editorsDigestive system disorders 2004 17th edUSASannders:590-91. [Google Scholar]
[11]. Byun J, Kim HY, Noh SY, Kim SH, Jung SE, Lee SC, Neonatal gastric perforation: A single centre experienceWorld J of Gastrointest Surg 2014 6(8):151-55.10.4240/wjgs.v6.i8.15125161763 [Google Scholar] [CrossRef] [PubMed]
[12]. Saracli T, Mann M, French DM, Booker CR, Scott RB, Rupture of the stomach in newborn infants Report of three cases and review of the world literatureClin Pediatr 1967 6(10):583-88.10.1177/0009922867006010146077501 [Google Scholar] [CrossRef] [PubMed]
[13]. Bruce J, Bianchi A, Doig CM, Gastric perforation in the neonatePediatr Surg Int 1993 8:17-19.10.1007/BF02352993 [Google Scholar] [CrossRef]
[14]. Stoll BJ, Hansen NI, Bell EF, Walsh MC, Carlo WA, Shankaran S, Trends in care practices, morbidity, and mortality of extremely preterm neonates, 1993-2012J Am Med Assoc 2015 314(10):1039-51. [Google Scholar]