The greatest risk factors enabling DIH reported in previous studies include HIV infection, hepatitis B or C infection [10-12,15,16], chronic liver disease, high alcohol consumption, malnutrition, advanced age, gender (female), and pregnancy [17]. However, there is no report and no proper knowledge regarding the risk factors that enhance the development of hepatotoxicity among patients with TB in Thailand.
The aim of the present study was to assess the risk factors enhancing the development of hepatotoxicity in patients with TB at one of the general hospitals in the North of Thailand from October 1st, 2016 to September 30th, 2018. The results of this study will support the development of appropriate surveillance and protective approaches regarding drug-induce hepatotoxicity, and also effective management for successful intensive treatment among TB patients with proper methods in Thailand.
Materials and Methods
Study design: A retrospective study was conducted to assess risk factors that enhance DIH development among patients with active TB during treatment for active TB at Phichit Hospital between October 1st, 2016 to September 30th, 2018. A total of 658 patients with active TB were identified by a physician, and cases with hepatotoxicity were identified by the American Thoracic Society criteria [18]. For this study, TB patients who were free from hepatotoxicity before starting anti-TB treatment were selected for this study and received routine follow-up for DIH symptoms evaluation by TB clinic team every day during their standard anti-TB regiment by phone in the first 2 months and every week in the last 4 months of their treatment. This study was reviewed and approved by the Ethics Committee for Research Involving Human Subjects Ethic of Phichit Hospital (PH-0034/2557). Informed consent was not required by the board because the data were collected from routine medical records and the results will be used for clinical purposes.
Recruitment Procedures
Inclusion criteria: New cases with TB who received the standard anti-TB regimen. The subjects in this study were 658 confirmed cases of TB diagnosed by a specific physician and 498 patients who had a positive Mycobacterium TB complex culture result according to an Acid-Fast Bacillus (AFB) smear either equal to or over those with negative culture result with clinical, histological and radiological features of TB, and whose condition improved on standard TB treatment, as was presumed. Finally, 327 patients with TB met eligible criteria. The standard anti-TB regimen in this study was defined as on the first two months, patients received 2HRZE/4HR, and the last four months they received HR.
Exclusion criteria: TB patients who were Multi-Drug Resistance (MDR), Extensively Drug-Resistant (XDR), Extremely Drug Resistant (XXDR), Totally Drug-Resistant (TDR), had an Alkaline Phosphatase (ALP) level > 3 times the Upper Normal Limit (UNL), had cirrhosis, chronic liver disease, bile duct cancer, liver cancer, were referred from another hospital, or did not receive continual treatment.
Anti-TB drug regimens, in this study were followed by National Tuberculosis Control Program Guideline of Thailand (2012) [19]:
Category 1=CAT 1: 2 (3) HRZE (S) / 4 HR
Category 2=CAT 2: 2 HRZES / 1(2) HRZE / 5 HRE
Category 3=CAT 3: 2HRZ/4 HR
Category 4=CAT 4: Used regimen with reserved drugs or INH alone
Note: Isoniazid(H) Rifampicin(R) Pyrazinamide(Z) Ethambutol (E) Streptomycin (S)
Anti-TB drug dosages, in this study were calculated in relation to the weight of Thai patients as follows [19]:
Rifampicin: Body weight 450-600 mg. (8-12 mg/kg/day)
Isoniazid: 300 mg (4-6 mg/kg/day)
Ethambutol: 800-1200 mg (15-20 mg/kg/day).
Pyrazinamide: 1000-2000 mg (20-30 mg/kg/day).
Streptomycin: (15 mg/kg/day).
Drug-Induced Hepatotoxicity (DIH): Patients who have increased Aspartate Amino Transferase (AST) or Alanine Transaminase (ALT) levels of more than three times the UNL who also have some hepatotoxicity symptoms such as being bored of food, being squeamish, vomiting, or jaundice; patients who have increased AST or ALT levels of more than 5 times the UNL without hepatotoxicity symptoms; or patients who have increased total bilirubin in the blood of more than 2 times the UNL without increased AST or ALT levels [8,20-22].
Chronic alcohol consumption: TB patients who have a history of high-level alcohol consumption or a consecutive alcohol intake of more than 48 g/day for more than one year during their treatment.
Hepatitis B or C infection: Patients who have a laboratory report showing HBsAg, anti-HCV antibodies.
HIV infection: Patients who have a laboratory report showing positive Anti-HIV.
Malnutrition: Patients with age more than 18 years who have a BMI of 18.5 kg/m2 and/or Albumin <3.5 g/dL [8,20].
Data Collection
The data were collected from the medical records program (HOSxP) and manual medical records of TB clinic in Phichit Hospital, Thailand. A record form was designed to collect information on patients with TB who received the standard anti-TB regimen and DIH. The information included demographic data, risk behaviours, medicines, symptoms, details of Anti-Tubercular Treatment (ATT), time of DIH onset from start of ATT, timing of reinstatement regimen and co-morbidity. Potentially enhancing factors of DIH including age, sex, baseline liver tests, HIV, hepatitis B and C, alcohol consumption, chronic liver disease and drug dosage per kg of body mass.
Study Variables for Enhancing Factors of Drug-induced Hepatotoxicity (DIH)
Independent variables (n=11) were included in the analysis, based on previous studies and were as follows [10-12,15,16]:
- Age group (>60 years vs ≤60 years)
- Sex (male vs female)
- BMI, kg/m2 (BMI <18.50 vs BMI 18.50-22.90 vs BMI >22.90)
- Malnutrition (yes vs no)
- HIV (yes vs no)
- Pregnancy (yes vs no)
- History of Hepatitis B and C (yes vs no)
- Alcohol consumption (yes vs no)
- Chronic liver disease (yes vs no)
- Co-morbidity (yes vs no)
- Drug dosage per kg of body mass. (lower dose vs proper dose vs over dose)
Statistical Analysis
For descriptive statistics, the baseline characteristics of the sample were used. The variables are represented as mean±SD (standard deviation) for continuous variables. Additionally, frequency and percentage are represented for categorical variables. For inferential statistics, analysis of risk factors which potentially enhance factors for DIH. Data analyses was done in 2 steps:
First step, bivariate analysis was performed to examine the degree of the association, separately in each categorical variables regarding DIH.
Second step, multivariate logistic regression model was constructed including the independent variables with p-value <0.15 results in bivariate analysis.
Odds Ratios (OR) were estimated and adjusted for baseline levels of ALT, ALP, and bilirubin as prior confounding factors.
Likelihood test was used for interaction consideration and Hosmer-Leme test showing goodness of fit was used for model fitness.
Software SPSS version 18.0 was used, and statistical significance was defined as a p-value of <0.05 with a 95% confidence interval.
Results
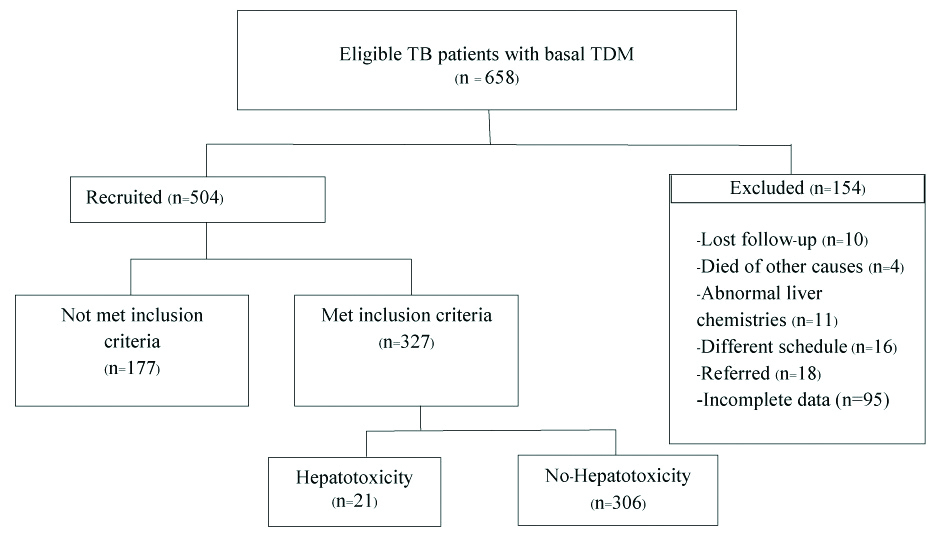
A total of 658 patients were treated with anti-TB treatment and followed-up their treatment from October 1st, 2016 to September 30th, 2018. The number of patients excluded from the study was 154. An additional 10 patients were not included due to a follow-up period of less than 8 weeks after anti-TB therapy; 4 died of other causes; 11 had underlying abnormal liver chemistries; 16 received medicine under a different dosage schedule; and 18 were referred to others hospitals. For 95 patients, there was incomplete imperative data for DIH assessment. Finally, 327 patients were included in this study, and among those, 21 patients (6.42%) developed hepatotoxicity [Table/Fig-1].
Flow chart showing recruitment of patients with Tuberculosis (TB).
TDM: Therapeutic drug monitoring
The mean age was 51±17.13 years (min=33 years, max=69 years). Most of them, 209 (63.90%), were men. Twenty-one cases (11 males, 10 females) developed hepatotoxicity and also, changed schedule of their regimen. The mean age was 51±17.13 years, 59.05±18.67 years in the hepatotoxicity group and 50.92±16.93 in no hepatotoxicity group. Among patients aged 60+ years, there were 112 cases (34.25%). Twelve cases (10.71%) developed hepatotoxicity, and 100 cases (89.29%) did not develop hepatotoxicity. In contrast, among patients aged <60 years, there were 215 cases (65.70%): 9 cases (4.19%) of hepatotoxicity and 206 cases (95.81%) with no hepatotoxicity. A total of 128 (39.14%) cases had a BMI <18.50 kg/m2, 11 cases (8.59%) had hepatotoxicity, and 117 cases (91.41%) had no hepatotoxicity [Table/Fig-2].
Demographic clinical and laboratory parametres of patients (n=327).
| Parameters | Total (n=327) | Hepatotoxicity (n=21) | No hepatotoxicity (n=306) |
|---|
| n (%) | n (%) | n (%) |
|---|
| Demographic and clinical characterstics |
| Gender |
| Male | 209 (63.90) | 11 (5.26) | 198 (94.74) |
| Female | 118 (36.10) | 10 (8.47) | 108 (91.53) |
| Age |
| (Years) | 51±17.13 | 59.05±18.67 | 50.92±16.93 |
| >60 Years | 112 (34.25) | 12 (10.71) | 100 (89.29) |
| ≤60 Years | 215 (65.70) | 9 (4.19) | 206 (95.81) |
| Height (cm) |
| Mean±SD | 162.23±8.26 | 165±10.90 | 162.12±8.18 |
| Weight (Kg) |
| Mean±SD | 51.95±10.00 | 50.61±13.41 | 52.05±9.67 |
| BMI (Kg/m2) |
| BMI <18.50 | 128 (39.14) | 11 (8.59) | 117 (91.41) |
| BMI 18.5-22.90 | 148 (45.26) | 10 (6.76) | 138 (93.24) |
| BMI >22.90 | 51 (15.60) | 0 (0.00) | 51 (100) |
| Laboratory parameters |
| Albumin (n) | 174 | 20 | 154 |
| (g/dL) | 3.39±0.66 | 2.93±0.78 | 3.45±0.63 |
| <3.50 n(%) | 84 (48.30) | 14 (16.67) | 70 (83.33) |
| ≥3.50 n(%) | 90 (51.70) | 6 (6.67) | 84 (93.33) |
| Total bilirubin (n) | 173 | 20 | 153 |
| (mg/dL) | 1.20±2.43 | 5.73±5.31 | 0.6±0.30 |
| ALT, (n) | 185 | 18 | 167 |
| (U/I) | 39.24±84.22 | 165.33±246.80 | 24.93±22.38 |
| AST, (n) | 185 | 18 | 167 |
| (U/I) | 142±117.85 | 285.47±330.55 | 35.36±22.03 |
| BUN (n) | 149 | 15 | 134 |
| (mg/dL) | 16.52±12.29 | 18.11±11.85 | 16.76±13.64 |
| Creatinine, (n) | 152 | 15 | 137 |
| mg/dL | 1.32±1.49 | 0.93±0.29 | 1.41±1.62 |
| CrCl (n) | 152 | 15 | 137 |
| (mL/min) | 79.46±34.32 | 80.66±23.23 | 79.33±35.39 |
| ≤30.00 (n) | 14 (9.20) | 0 (0.00) | 14 (100.00) |
| >30.00 (n) | 138 (90.80) | 15 (10.87) | 123 (89.13) |
*BUN: Blood urea nitrogen; BMI: Body mass index, ALT: Alanine transaminase, AST: Aspartate aminotransferase, CrCl: Creatinine clearance
The mean of albumin in the blood among 174 patients was 3.39±0.66 g/dL, 2.93±0.78 g/dL in the hepatotoxicity group and 3.45±0.63 g/dL in the no hepatotoxicity group. Fourteen cases (16.67%) in the patients with albumin levels of less than 3.5 g/dL group had hepatotoxicity. The total bilirubin in the hepatotoxicity group was 5.73±5.31 mg/dL and 0.6±0.30 mg/dL in those with no hepatotoxicity. The mean of ALT in the hepatotoxicity group was 165.33±246.80 U/l and 24.93±22.38 U/l in those with no hepatotoxicity. The AST level in the hepatotoxicity group was 285.47±330.550 U/l and 35.36±22.03 U/l in those with no hepatotoxicity. The BUN level in the hepatotoxicity group was 18.11±11.85 mg/dL and 16.76±13.64 mg/dL in those with no hepatotoxicity. Creatinine in the hepatotoxicity group was 0.93±0.29 mg/dL and 1.41±1.62 mg/dL in those with no hepatotoxicity. Creatinine clearance in the hepatotoxicity group was 80.66±23.23 mL/min and 79.33±35.39 mL/min in those with no hepatotoxicity and no case with Creatinine clearance ≤30.00 mL/min in the hepatotoxicity group [Table/Fig-2].
The results of the comparison between hepatotoxicity group and no hepatotoxicity group found that ten cases (50.00%) with chronic alcohol consumption had hepatotoxicity, and no cases with hepatitis B or C had hepatotoxicity. However, three cases (15.79%) without a history of hepatitis B or C had hepatotoxicity. Two cases (6.90%) who were HIV positive showed hepatotoxicity. Only one TB patients with pregnancy in this study had no-hepatotoxicity. Eleven patients (11.34%) with hepatotoxicity had underlying diseases; most of them had hypertension and diabetes. There were three cases (9.38%) with a low dosage regimen and one case (25.00%) with a proper dosage of a single drug regimen that showed hepatotoxicity. In addition, 13 cases (5.24%) with proper dosage and two cases (13.33%) with high dosage of a mixed regimen that showed hepatotoxicity [Table/Fig-3].
Health status and health risk behaviours of TB patients (n=327).
| Parameters | Total (n=327) | Hepatotoxicity (n=21) | No hepatotoxicity (n=306) |
|---|
| Pregnancy (n) | 118 | 10 | 108 |
| Yes | 1 (0.85) | 0 (0.00) | 1 (100.00) |
| No | 117 (99.15) | 10 (8.55) | 107 (91.45) |
| Malnutrition (n) | 230 | 20 | 210 |
| Yes | 64 (27.82) | 16 (25.00) | 48 (75.00) |
| No | 166 (72.18) | 4 (2.41) | 162 (97.59) |
| Hepatitis B, C (n) | 27 | 3 | 24 |
| Yes | 8 (29.60) | 0 (0.00) | 8 (100.00) |
| No | 19 (70.40) | 3 (15.79) | 16 (84.21) |
| Anti-HIV (n) | 256 | 15 | 241 |
| Positive | 29 (11.30) | 2 (6.90) | 27 (93.10) |
| Negative | 227 (88.70) | 13 (5.73) | 214 (94.27) |
| Underlying disease (n) | 327 | 21 | 306 |
| Yes | 97 (29.70) | 11 (11.34) | 86 (88.66) |
| No | 230 (70.30) | 10 (4.35) | 220 (95.65) |
| Underlying disease (n) | 165 | 18 | 147 |
| Diabetes | 35 (21.22) | 2 (5.71) | 33 (94.29) |
| Hypertension | 53 (32.12) | 7 (13.21) | 46 (86.79) |
| Cancer | 2 (1.21) | 0 (0.00) | 2 (100.00) |
| Cardiovascular disease | 2 (1.21) | 0 (0.00) | 2 (100.00) |
| Others | 73 (42.24) | 9(12.33) | 64 (87.67) |
| Chronic alcohol consumption (n) | 30 | 10 | 20 |
| Yes | 20 (66.70) | 10 (50.00) | 10 (50.00) |
| No | 10 (33.30) | 0 (0.00) | 10 (100.00) |
| Regimens (n) | 314 | | |
| HRZE (Single) (n) | 38 | 4 | 34 |
| Lower dose | 32 (84.20) | 3 (9.38) | 29 (90.62) |
| Proper dose (n) | 4 (10.50) | 1 (25.00) | 3 (75.00) |
| Over dose | 2 (5.30) | 0 (0.00) | 2 (100.00) |
| HRZE (Mixed) (n) | 276 | 16 | 260 |
| Lower dose | 13 (4.70) | 1 (7.69) | 12 (92.31) |
| Proper dose | 248 (89.90) | 13 (5.24) | 235 (94.76) |
| Over dose | 15 (5.40) | 2 (13.33) | 13 (86.67) |
*HRZE=Isoniazid (H), Rifampicin (R), Pyrazinamide (Z), Ethambutol (E), Streptomycin (S), TB: Tuberculosis
This study found that most patients with TB who received the standard regimen (HRZE) had pulmonary TB (231, 70.64%) and most of hepatotoxicity cases were pulmonary TB, (15, 4.59%)[Table/Fig-4].
Details of site of TB classified by Hepatotoxicity (n=327).
| Site of TB | Cases of present study (n=327) | Hepatotoxicity (n=21) | No hepatotoxicity (n=306) |
|---|
| Pulmonary system | 231 (70.64) | 15 (4.59) | 216 (66.06) |
| Pleura | 16 (4.89) | 4 (1.22) | 12 (3.67) |
| Lymph nodes | 35 (10.70) | 2 (0.61) | 33 (10.09) |
| Peritoneum | 4 (1.22) | 0 (0.00) | 4 (1.22) |
| Spine | 22 (6.73) | 0 (0.00) | 22 (6.73) |
| Pericardium | 2 (0.62) | 0 (0.00) | 2 (0.61) |
| Other | 17 (5.20) | 0 (0.00) | 17 (5.20) |
TB: Tuberculosis
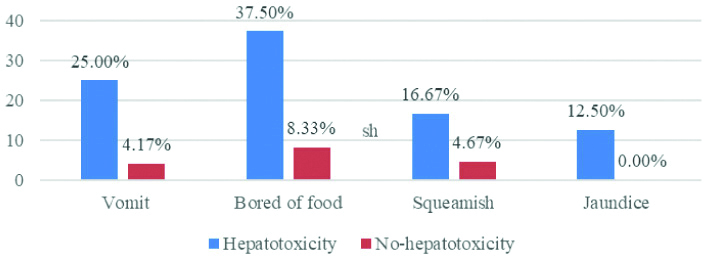
The most common symptom found in TB patients who received the standard regimen was being bored of food in both hepatotoxicity and no hepatotoxicity groups. However, this study discovered that patients with hepatotoxicity showed a higher percentage for each symptom including vomiting, being bored of food, being squeamish, and especially having jaundice, which was not found in the no hepatotoxicity group [Table/Fig-5].
Percentage of symptoms among TB patients who received the standard drug regimen (n=24).
The potentially risk factors enhancing DIH development were used for analysis in this model, including age group, malnutrition, HIV infection, history of hepatitis B or C infection, chronic alcohol consumption, and pregnancy. There were other factors that were considered direct risk factors of DIH in patients with TB, such as treatment regimen, drug, and dosage of drugs. In addition, the baseline factors including albumin, ALT, AST, BUN, and creatinine were not used to examine enhancing risk factors because they were used for identification of DIH. They are not risk factors; instead, they are the effects from other risk factors.
The results of this study confirmed that malnutrition was positively associated with increased risk of DIH development (adjusted OR 4.40 (1.26 to 12.26), p=0.01) [Table/Fig-6]. In addition, an age of >60 years was associated with an increased risk of DIH development (adjusted OR 2.87 (95% CI 1.16 to 7.14), p=0.02). According to the data from the small number of HIV infected patients, HIV infection was not associated with an increased risk of DIH development (adjusted OR 0.96 (95% CI 0.21 to 6.10), p=0.70).
Risk factors enhancing drug-induced hepatotoxicity development: univariate and multivariate analysis.
| Factors | Hepatotoxicity | Univariate analysis | p-value | Multivariate analysis | p-value |
|---|
| n/N | % | OR | 95% CI | adj. OR | 95% CI |
|---|
| Age, (n=327) |
| >60 years | 12/21 | 57.14 | 2.747 | 1.12 to 6.73 | 0.02 | 2.87 | 1.16 to 7.14 | 0.02 |
| ≤60 years | 9/21 | 42.86 |
| HIV infection (n=256) |
| Yes | 2/15 | 13.33 | 1.219 | 0.26 to 5.69 | 0.68 | 0.96 | 0.21 to 6.10 | 0.70 |
| No | 13/15 | 86.66 |
| Malnutrition (n=230) |
| Yes | 16/20 | 80.00 | 3.778 | 1.22 to 11.67 | 0.01 | 4.40 | 1.26 to12.36 | 0.01 |
| No | 4/20 | 20.00 |
Adjusted for gender and baseline ALT, ALP, and bilirubin; Hosmer lemeshow test Chi-square, p-value=0.068
No positive association was found between DIH and history of hepatitis B or C infection, chronic alcohol consumption, or pregnancy; all of which were not associated with DIH development among patients with TB who received the standard anti-TB regimen.
Discussion
The results confirmed that patients with TB who were >60 years of age will be at risk of DIH; this result was similar to the prospective case-control study [21] and other previous studies [4,9], which found that an age of 60+ years was a specific risk factor for hepatotoxicity because the kidney function for eliminating toxins is not effective in elderly persons; therefore, toxins in the blood increasingly collect in the liver [20]. This effect is a major cause of DIH, especially in patients with high drug dosages, such as patients with TB [14] and also high age person may be due to enlarged prevalence of co-morbidity conditions as well as use of related additional drugs in this age group [22]. However, this result contrasts with a previous retrospective study from a large TB center in the UK [23], which found no positive association between increasing age and risk of DIH. Consequently, patients with TB in Thailand should receive liver function tests such as AST and ALT, before receiving the TB treatment and these are potentially devices for hepatotoxicity surveillance among patients with TB who received the standard anti-TB regimen.
The current study found that patients with a BMI <18.5 kg/m2 or Albumin <3.5 g/dL were at high risk to get DIH; this is consistent with previous studies [19,23], which reported that malnutrition is common in TB patients and associated with a higher incidence of anti-TB DIH [20]. A previous retrospective observational study showed that a weight loss of 2 kg or more within 4 weeks during TB treatment is a highly significant independent risk factor for anti-TB drug induced hepatotoxicity [21]. A sufficient intake of nutrients is essential for the integrity of liver metabolism and its ability to detoxify TB drugs, considering that the cytochrome P450 enzyme system is altered by nutrient intake, fasting, and malnourishment [24].
However, HIV infection, Hepatitis B or C, and alcohol intake were not found to be positive risk factors, which is consistent with previous study [10-12,15,16], which reported that a small number (eight) of HIV-1 co-infected patients had a four-fold higher risk of DIH and adverse events that often complicated the course of anti-tuberculous treatment and the outcome of treatment get worse due to advanced immunodeficiency [11]. These results may be affected by the fact that there were only a small number of patients with HIV infection in this study.
This study showed that Hepatitis B or C were not associated with DIH development among TB patients with standard anti-TB regimen. The findings of the present study were not comparable to previous studies, which confirmed that Hepatitis B and C were potential risk factors of hepatotoxicity [9,10,15,16]. The likelihood that there were low occurrence of Hepatitis B and C among the TB patients in this study. Therefore, we did not found the existent effect of Hepatitis B and C to hepatotoxicity in this study.
In addition, excessive alcohol use has been reported as a risk factor of hepatotoxicity in a previous study [22]. The possibility that in this study, we report alcohol consumption, and also pregnancy and co-morbidity as risk factors given the low incidence. Consequently, we did not found the existent association in the present study.
Thus, the occurrence of potentially risk factors which affect DIH development among patient with TB should be considered for further studies.
Limitation(s)
It was retrospective study, which means that monitoring for occurrence of DIH may not have been consistent over time. However, this is unlikely considering that the TB specialists managing patients were the same during this period. This would also have contributed to incomplete recording of symptoms. In addition, the occurrence of some variables were low, such as HIV infection, alcohol consumption and pregnancy, which caused difficulty during statistical analysis and interpretation for consistent results.
Conclusion(s)
This study has provided information that in TB patients, an age of >60 years and malnutrition are enhancing factors of DIH. The information from this study can be used for conducting the DIH surveillance approaches in Thai patients with TB who received the standard anti-TB regimen. Therefore, the new patients with TB should be received the screening for present chronic liver disease, including Aspartate Amino Transferase (AST) and Alanine Transaminase (ALT) whenever feasible, and nutritional assessment should be done for elderly patients before starting anti-TB treatment.
*BUN: Blood urea nitrogen; BMI: Body mass index, ALT: Alanine transaminase, AST: Aspartate aminotransferase, CrCl: Creatinine clearance
*HRZE=Isoniazid (H), Rifampicin (R), Pyrazinamide (Z), Ethambutol (E), Streptomycin (S), TB: Tuberculosis
TB: Tuberculosis
Adjusted for gender and baseline ALT, ALP, and bilirubin; Hosmer lemeshow test Chi-square, p-value=0.068