Cavernous Sinus Metastasis from Carcinoma of Buccal Mucosa- A Rare Case Report and Review of Literature
Sambit S Nanda1, Ayushi Patni2, Ajeet K Gandhi3, Madhup Rastogi4, Satyajeet Rath5
1 Senior Resident, Department of Radiation Oncology, Dr. Ram Manohar Lohia Institute of Medical Sciences, Lucknow, Uttar Pradesh, India.
2 Junior Resident, Department of Radiation Oncology, Dr. Ram Manohar Lohia Institute of Medical Sciences, Lucknow, Uttar Pradesh, India.
3 Assistant Professor, Department of Radiation Oncology, Dr. Ram Manohar Lohia Institute of Medical Sciences, Lucknow, Uttar Pradesh, India.
4 Professor, Department of Radiation Oncology, Dr. Ram Manohar Lohia Institute of Medical Sciences, Lucknow, Uttar Pradesh, India.
5 Senior Resident, Department of Radiation Oncology, Dr. Ram Manohar Lohia Institute of Medical Sciences, Lucknow, Uttar Pradesh, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Ayushi Patni, Department of Radiation Oncology, Dr. Ram Manohar Lohia Institute of Medical Sciences, Vibhuti Khand, Gomti Nagar, Lucknow, Uttar Pradesh, India.
E-mail: ayushipatni2394@gmail.com
Distant metastasis from Head and Neck Squamous Cell Carcinomas (HNSCC) is uncommon, Cavernous Sinus (CS) metastasis being very rare. Infrequent presentation and misdiagnosis makes it a difficult entity to identify and treat. Hereby, Authors present a rare case of 47 year old male of CS metastasis from postoperative carcinoma of buccal mucosa. The patient was a histopathologically proven case of poorly differentiated Squamous Cell Carcinoma (SCC) of left buccal mucosa for which he underwent definitive surgery. During adjuvant Radiotherapy (RT) he developed severe headache, diplopia and ptosis. 18Fluorodeoxy-Glucose-Positron Emission Tomography-Computed Tomography (18FDG PET-CT) revealed an FDG avid lesion in left CS suggestive of CS metastasis. In view of local recurrence and CS metastasis palliative RT was given. Patient responded well to palliative RT but succumbed to the disease within months. CS metastasis has poor prognosis with limited treatment options which include palliative RT, either External Beam Radiotherapy (EBRT) or radiosurgery to CS. Chemotherapy for disseminated disease has limited benefits due to lesser penetration of the blood brain barrier.
Cavernous sinus syndrome, Metastasis in oral cavity carcinoma, Oral cavity carcinoma with distant metastasis
Case History
A 47-year-old male, chronic tobacco chewer, without any medical co-morbidities presented with a non-healing ulcer in left buccal mucosa for two months. On clinical examination, an ulcero-proliferative growth of 3×2.5 cm was seen in the left buccal mucosa extending to left superior and inferior gingivobuccal sulcus. A 1.5×1.5 cm hard, mobile, non-tender lymph node was also noted in left side of neck (level Ib). Computed Tomography (CT) scan of face and neck revealed an ulcero-proliferative lesion, 3.7×2.2×3.3 cm in size, in left buccal mucosa extending to the superior and inferior gingivobuccal sulci and retromolar trigone without involvement of the pterygoid or temporalis muscles. Multiple enlarged lymph nodes noted at left level Ib largest measuring 1.6×1.4 cm. A provisional diagnosis of carcinoma of left buccal mucosa was made and staged as cT2N2bM0 (AJCC 7th edition) [1].
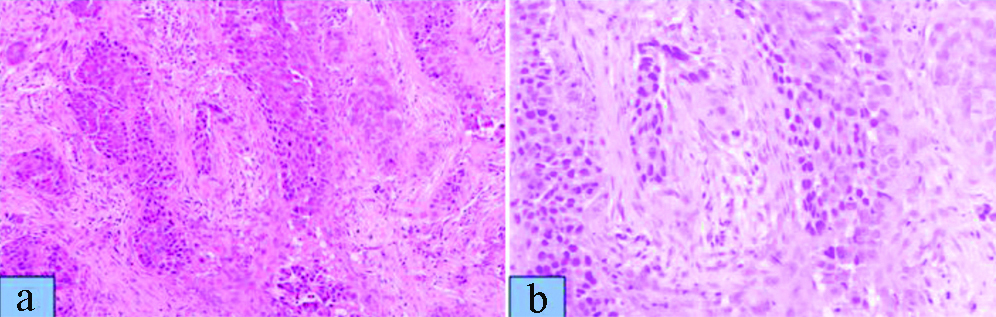
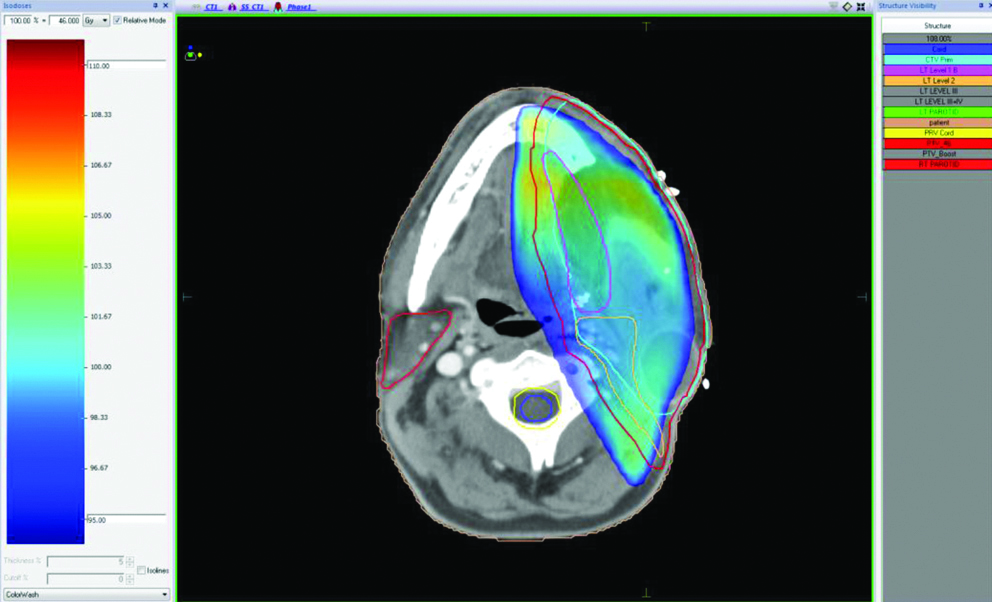
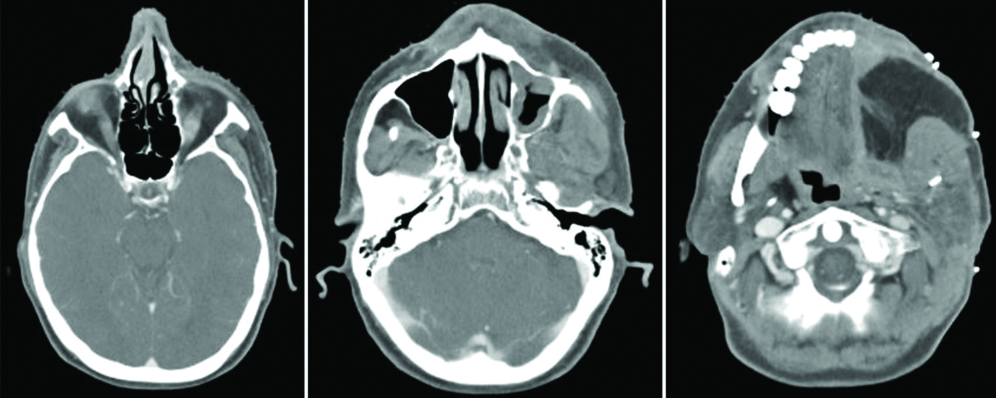
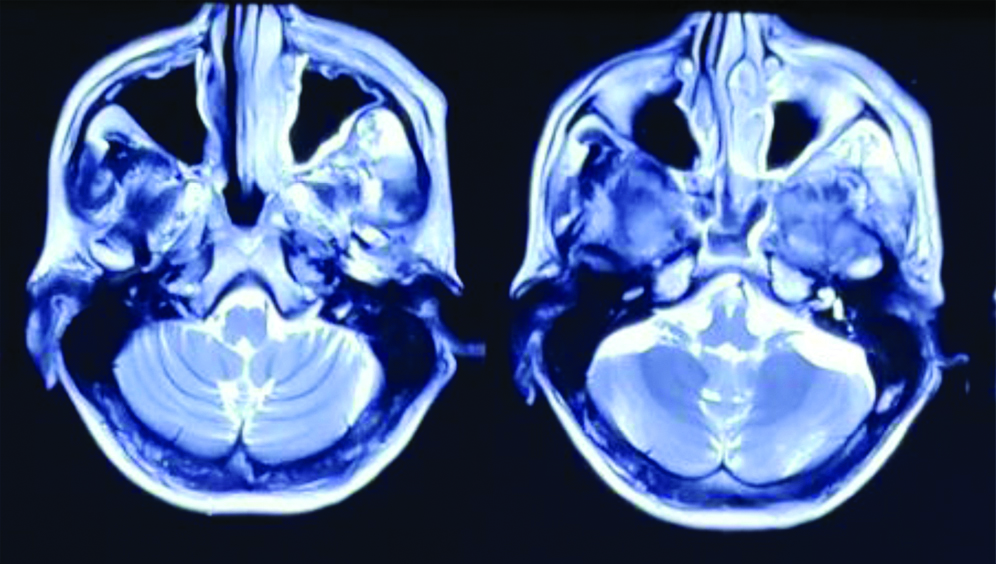
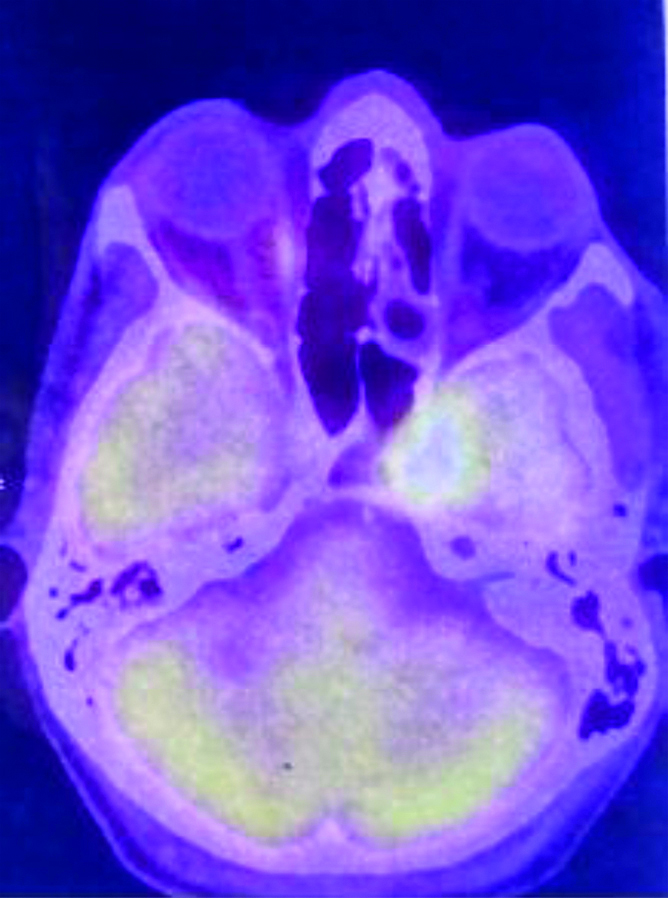
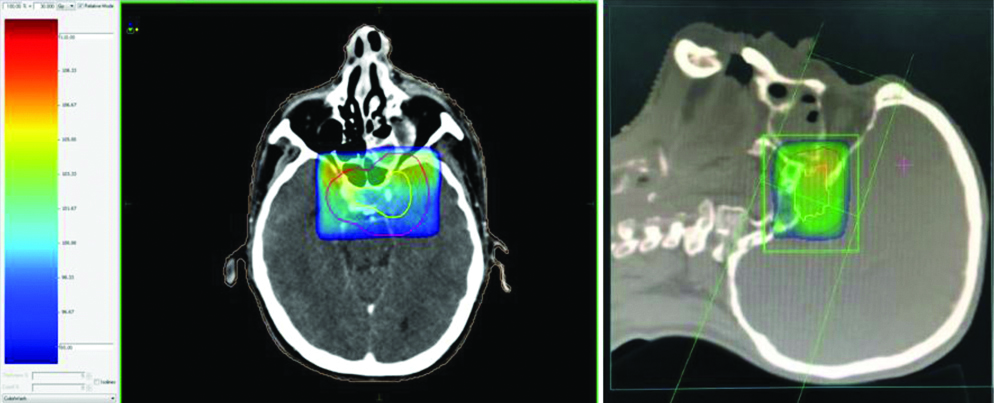
Punch biopsy showed disordered cells with abundant nuclear pleomorphism suggestive of poorly differentiated SCC [Table/Fig-1]. The patient underwent wide local excision of left buccal mucosa with marginal mandibulectomy and left modified radical neck dissection. Postoperative histopathology showed 2.5×1.5×1.5 cm poorly differentiated SCC with depth of invasion 5 mm, clear margins with uninvolved overlying skin and bone. Perineural invasion was present without lympho-vascular invasion. Two out of 32 lymph nodes were positive with gross involvement on histopathological examination without extra-nodal extension and disease was staged as pT2N2bM0 (AJCC 7th). Patient was planned for adjuvant RT 64 Gray in 32 fractions over 6.5 weeks, 46 Gray in 23 fractions over 4.5 weeks was planned to tumour bed, ipsilateral nodes (level I-IV) [Table/Fig-2], followed by 18 Gray boost. Planning contrast enhanced CT scan did not reveal any recurrence of disease in postoperative bed, neck or CS [Table/Fig-3]. After 23 fractions, he started complaining of ipsilateral severe frontal headache and diplopia and on examination patient had ptosis and ipsilateral ophthalmoplegia suggestive of 3rd cranial nerve involvement which progressed rapidly over a period of 4-5 days. He did not show any other features of raised intracranial tension or diminishing vision. Magnetic Resonance Imaging (MRI) brain showed intermediate signal intensity lesion on T2W images in left CS encasing the Internal Carotid Artery and initially a diagnosis of CS thrombosis was suspected [Table/Fig-4]. 18FDG- PET-CT revealed a hypermetabolic (SUVmax-6.22) lesion in superior border of surgical margin in upper gingivobuccal sulcus with destruction of anterolateral wall of left maxillary sinus extending into right temporal fossa and another FDG avid (SUVmax-7.56) lesion in left CS eroding clinoid process and extending into pituitary fossa [Table/Fig-5]. Lumbar puncture for cerebrospinal fluid cytology was negative for malignant cells. In view of local recurrence and CS metastasis adjuvant RT was interrupted and patient was given palliative RT encompassing CS to the dose of 30 Gray in 10 fractions [Table/Fig-6]. He responded well to this and by the end of palliative RT, diplopia and ophthalmoplegia subsided completely and subjective pain relief was 80% as per Visual Analogue Scale. Option of palliative chemotherapy was discussed with patient but was deferred in view of his refusal. Patient was kept on best supportive care but succumbed to the disease within three months of diagnosis of CS metastasis.
Discussion
HNSCC is the most common type of cancer amongst males in India, with oral cavity being the most common subsite. Oral cancer accounts for around 30% of all cancers in India [2]. HNSCC is notorious for loco-regional recurrence. Distant spread is uncommon but may be seen in lungs, bone, liver with infrequent involvement of brain. Metastasis to CS from HNSCC is very rare. CS metastases have been reported in a few cases of primary tumours of head and neck like oropharynx and larynx [3-5]. Infrequent presentation and misdiagnosis of CS metastasis from HNSCC makes it a difficult entity to identify and treat.
The CS are paired, venous structures located on either side of the sella turcica which receive venous tributaries from the superior and inferior orbital veins and drain into the superior and inferior petrosal sinuses. CS contains the carotid artery, its sympathetic plexus, and the oculomotor (third), trochlear (fourth), ophthalmic branch of trigeminal (fifth) and abducent (sixth) cranial nerves. Thus, involvement of CS presents as headache, ophthalmoplegia, diplopia, retro-orbital pain, proptosis, ocular and conjunctival congestion, ocular hypertension, dysesthesia in face and ptosis [6]. These features can be seen in various clinical conditions like CS tumours, aneurysms, thrombosis, carotid-cavernous fistulas [7]. Neoplastic lesions in CS may be primary tumours (meningioma, neurofibroma), perineural invasion of head and neck cancers [8] or haematogenous spread from distant sites.
Radiology remains the cornerstone for diagnosis of CS pathology like meningioma, thrombosis, aneurysm and metastasis all of which tend to present in a similar fashion. Contrast enhanced MRI remains the gold standard to detect CS metastasis and usually shows CS enlargement, outward bowing of lateral wall, and replacement of the meckel cave with homogenously enhancing soft tissue. Perineural tumour spread is commonly seen along the branches of trigeminal cranial nerve as nerve enlargement and enhancement [9]. In case of a known primary malignancy, a suspected lesion of the CS with clinical and radiological findings should be considered metastatic until proven otherwise. Histopathological confirmation is difficult and rarely trans-sphenoidal or sub-temporal biopsy can be done. Differential diagnosis includes Cavernous haemangioma, which are hyperintense on T1- and T2-weighted images and show progressive post contrast “filling in”. CS meningioma enhances intensely and is hypo- to isointense in all MRI sequences. Subacute CS thrombosis exhibits high signal intensity on MRI whereas acute thrombosis may be isointense [9].
CS metastasis portends a dismal outcome. In view of poor blood brain barrier penetration of most active chemotherapeutic agents, palliative RT remains the mainstay of treatment. The effect of RT is dose dependant with limiting factors being size of the tumour, adjoining organs at risk and extent of cranial nerve involvement [10]. RT can be given either as external beam RT (20-30 Gray/5-10 fractions) or radiosurgery can be done in cases of controlled primary with a margin dose of 15-20 Gray [11]. Radiosurgery is effective in alleviating symptoms with sparing of surrounding normal tissues thus it has lesser neurological deficits. Gamma knife radiosurgery with a sharp dose fall-off profile may better spare normal tissues. Surgical resection for CS metastasis is not usually indicated as new chemotherapy drugs and radiosurgery and can achieve good local control with neurological recovery without surgical risks. There have been few case reports and series pertaining to CS metastasis. González García R et al., reported a CS metastasis from oropharynx, which was treated with 57 Gray palliative RT providing good symptomatic palliation [3]. Traserra J et al., in one of the earliest series reported three patients (2 larynx, 1 hypopharynx) of CS metastasis, with two of them treated with palliative RT with dismal outcome [4]. Similarly Zhu J et al., treated CS metastasis from face with palliative paclitaxel and carboplatin based chemotherapy and 37 Gray palliative RT [7]. Recurrence of malignancy in CS after 10 years in a treated case of acinic cell carcinoma of parotid gland has also been reported which was treated with palliative RT 30 Gray in 10 fractions and chemotherapy (3 weekly paclitaxel and carboplatin). Patient showed partial response at 12 months [12]. Another case report of CS metastasis from castrate-resistant metastatic prostate cancer was treated with 20 Gray in 5 fractions to the CS mass with symptomatic improvement [13]. Despite different approaches overall prognosis of CS metastasis remains poor. Survival reported with various treatment modalities in CS metastasis from different primary sites is shown in [Table/Fig-7] [10,11,14-17]. Further studies are warranted to determine more efficient chemotherapy drugs and RT technique to optimise outcome.
a) Shows poorly differentiated squamous cell carcinoma with surrounding inflammatory infiltrate and stromal desmoplasia (H&E X10); b) Higher magnification of same showing marked nuclear pleomorphism (H&E X100).
Planning of adjuvant radiotherapy with isodose gradient.
Axial images of different levels of planning contrast enhanced CT scan.
T2W images on MRI brain showing intermediate signal intensity lesion in left cavernous sinus encasing the internal carotid artery suggestive of cavernous sinus thrombosis.
18FDG PET-CT showing FDG avid (SUVmax-7.56) lesion in left cavernous sinus eroding clinoid process and extending into pituitary fossa suggestive of cavernous sinus metastasis.
Axial and sagittal images of radiotherapy planning of palliative radiotherapy for cavernous sinus metastasis with isodose gradient.
Survival reported with various treatment modalities in cavernous sinus metastasis in literature [10,11,14-17].
| S. No. | Author (year) | No. of patients with cavernous sinus metastasis | Treatment modality (Margin tumour dose) | Follow-up (FU) | Outcome |
|---|
| 1. | Ayer A et al., (2014) [14] | N=19 | Gamma knife stereotactic radiosurgery (18 Gy) | Median FU=22.4 months | Overall survival at 1 y-76%2 y-44%4 y-44% |
| 2. | Kano H et al., (2009) [11] | N=37 | Stereotactic radiosurgery (14 Gy) | Mean FU=12.9 months | Overall survival at 1 y-36.6%2 y-19.4% |
| 3. | Mori Y et al., (2006) [15] | N=9 | Gamma knife surgery (14.4-20 Gy) | Median FU=4 months | Stable-3Complete or partial response-6 |
| 4. | Iwai Y et al., (2005) [10] | N=21 | Gamma knife surgery (14 Gy) | Median FU=9 months | Mean overall survival-13 months |
| 5. | Iwai Y and Yamanaka K (1999) [16] | N=18 | Gamma knife radiosurgery (16.2 Gy) | Median FU=10.5 months | Mean overall survival-14.1 months |
| 6. | Bumpous JM et al., (1993) [17] | N=6 | Radiotherapy-3, Chemotherapy-1 (3 patients diagnosed at autopsy) | - | Mean survival-4 months |
Conclusion(s)
Cavernous Sinus metastasis in SCC of buccal mucosa is rare and portends a very poor prognosis. Signs and symptoms like severe headache, ophthalmoplegia, retro-orbital pain, diplopia, and ptosis should raise a suspicion of CS involvement which can be appreciated on contrast enhanced MRI brain. Palliation can be achieved with RT and chemotherapy but survival remains poor.
Author Declaration:
Financial or Other Competing Interests: None
Was informed consent obtained from the subjects involved in the study? Yes
For any images presented appropriate consent has been obtained from the subjects. Yes
Plagiarism Checking Methods: [Jain H et al.]
Plagiarism X-checker: Apr 13, 2020
Manual Googling: Jun 12, 2020
iThenticate Software: Aug 22, 2020 (7%)
[1]. Edge SB, Compton CC, The American Joint Committee on Cancer: The 7th edition of the AJCC cancer staging manual and the future of TNMAnn Surg Oncol 2010 17(6):1471-74.10.1245/s10434-010-0985-420180029 [Google Scholar] [CrossRef] [PubMed]
[2]. Oral Cancer [Internet]. India Against Cancer. 2020. Available from: http://cancerindia.org.in/oral-cancer [Google Scholar]
[3]. González García R, Sastre Pérez J, Naval Gías L, Rodríguez Campo FJ, Díaz González FJ, Cavernous sinus metastasis from oropharyngeal squamous cell carcinomaMed Oral Patol Oral Cir Bucal 2007 12(2):E166-70. [Google Scholar]
[4]. Traserra J, Comas J, Conde C, Cuchi A, Cardesa A, Metastatic involvement of the cavernous sinus from primary pharyngolaryngeal tumoursHead Neck 1990 12(5):426-29.10.1002/hed.28801205102211104 [Google Scholar] [CrossRef] [PubMed]
[5]. Curry M, Newlon J, Watson D, Cavernous sinus metastasis from laryngeal squamous cell carcinomaOtolaryngol Head Neck Surg 2001 125(5):567-68.10.1067/mhn.2001.11678111700465 [Google Scholar] [CrossRef] [PubMed]
[6]. Shelton J, Ramakrishnaiah R, Glasier C, Phillips P, Cavernous sinus syndrome from an internal carotid artery aneurysm in an infant with tuberous sclerosisJ AAPOS 2011 15(4):389-91.10.1016/j.jaapos.2011.03.01321816642 [Google Scholar] [CrossRef] [PubMed]
[7]. Zhu J, Padillo O, Duff J, Hsi BL, Fletcher JA, Querfurth H, Cavernous sinus and leptomeningeal metastases arising from a squamous cell carcinoma of the face: Case reportNeurosurgery 2004 54(2):492-99.10.1227/01.NEU.0000103674.30974.6914744296 [Google Scholar] [CrossRef] [PubMed]
[8]. Lee J, Lee H, Park J, Choi CG, Suh DC, Cavernous sinus syndrome: Clinical features and differential diagnosis with MR imagingAJR Am J Roentgenol 2003 181(2):583-90.10.2214/ajr.181.2.181058312876052 [Google Scholar] [CrossRef] [PubMed]
[9]. Belsuzarri T, Seixas N, Belsuzarri N, Pozetti M, Araujo JFM, Cavernous sinus syndrome as the first manifestation of metastatic breast diseaseSurg Neurol Int 2017 8(1):4010.4103/sni.sni_359_1628480103 [Google Scholar] [CrossRef] [PubMed]
[10]. Iwai Y, Yamanaka K, Yoshimura M, Gamma knife radiosurgery for cavernous sinus metastases and invasionSurg Neurol 2005 64(5):406-10.10.1016/j.surneu.2004.12.02116253685 [Google Scholar] [CrossRef] [PubMed]
[11]. Kano H, Niranjan A, Kondziolka D, Flickinger JC, Lunsford LD, The role of palliative radiosurgery when cancer invades the cavernous sinusInt J Radiat Oncol Biol Phys 2009 73(3):709-15.10.1016/j.ijrobp.2008.05.00518692328 [Google Scholar] [CrossRef] [PubMed]
[12]. Francis Thottian AG, Gandhi AK, Ramateke PP, Gogia A, Acinic cell carcinoma of parotid gland with cavernous sinus metastasis: A case reportJ Can Res Ther 2018 14:1428-30. [Google Scholar]
[13]. Kuiper B, Babikian A, Delacruz W, Metastatic prostate cancer manifesting as cavernous sinus syndrome-Case report and review of the literatureOncology & Hematology Review 2017 13(1):59-63.10.17925/OHR.2017.13.01.59 [Google Scholar] [CrossRef]
[14]. Ayer A, Page BR, Lucas Jr JT, Bourland JD, Oliver ER, Tatter SB, Cavernous sinus metastases treated with gamma knife TM stereotactic radiosurgeryJ Radiosurg SBRT 2014 3(2):131 [Google Scholar]
[15]. Mori Y, Kobayashi T, Shibamoto Y, Stereotactic radiosurgery for metastatic tumours in the pituitary gland and the cavernous sinusJ Neurosurg 2006 105(Supplement):37-42.10.3171/sup.2006.105.7.3718503328 [Google Scholar] [CrossRef] [PubMed]
[16]. Iwai Y, Yamanaka K, Gamma Knife radiosurgery for skull base metastasis and invasionStereotact Funct Neurosurg 1999 72(Suppl. 1):81-87.10.1159/00005644310681695 [Google Scholar] [CrossRef] [PubMed]
[17]. Bumpous JM, Maves MD, Gomez SM, Levy BK, Johnson F, Cavernous sinus involvement in head and neck cancerHead Neck 1993 15(1):62-66.10.1002/hed.28801501148416860 [Google Scholar] [CrossRef] [PubMed]