Gestational Diabetes Mellitus and its Relation to Pre-pregnancy Body Mass Index
Veena Thamban1, Kavana G Venkatappa2
1 Assistant Professor, Department of Physiology, Government Medical College, Kannur, Kerala, India.
2 Associate Professor, Department of Physiology, Government Medical College, Kannur, Kerala, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Veena Thamban, Near Canara Bank, Raja Road Nileshwar (P.O), Kasargod, Kerala-671314, Kannur, India.
E-mail: dr.veenanlr@gmail.com
Introduction
Obesity mediates a systemic inflammatory response in our body which includes insulin resistance and glucose dysregulation. Increased Body Mass Index (BMI) associated with Gestational Diabetes Mellitus (GDM) leads to a state of insulin resistance additive to insulin resistance of GDM.
Aim
To assess the relationship between GDM and pre-pregnancy BMI.
Materials and Methods
This case-control study enrolled 64 women with GDM and 64 without GDM, attending antenatal care clinic at a Tertiary Care Hospital, after obtaining their informed consent. Obstetric history, pre-pregnancy BMI and Oral Glucose Tolerance Test (OGTT) values were noted. Diabetes in Pregnancy Study Group in India (DIPSI) criterion was used for diagnosing GDM. The BMI was categorised according to World Health Organisation (WHO) criterion. Data obtained was statistically analysed.
Results
Out of 44, 41 (93.2%) women with GDM had pre-pregnancy BMI ≥25 kg/m2 (overweight and obese) compared to 3 (6.8%) in controls (χ2 value=50.01, p≤0.001). Mean±SD of pre-pregnancy BMI in women with GDM was significantly higher (26.38±2.74 kg/m2) compared to controls i.e., 22.26±1.54 kg/m2 (unpaired t-test: p≤0.001).
Conclusion
GDM was found to be significantly associated with pre-pregnancy BMI. Appropriate interventions and risk factor modifications are recommended to prevent GDM and its complications.
Insulin, Obesity, Pregnancy, Prevalance, Risk factor
Introduction
The GDM is a global health concern as it affects health status of both mother and fetus. GDM refers to any degree of glucose intolerance with onset or first recognition during pregnancy [1]. Affects 1-14% of all pregnancies and represents nearly 90% of all pregnancies complicated by diabetes [2]. The increased prevalence of GDM is contributed by the obesity epidemic, urbanisation, aging population structure and physical inactivity. Among ethnic groups in South Asian countries, South Indian women have the highest frequency of GDM [3].
In general, obesity is an important predisposing factor for the development of diabetes mellitus and GDM in specific. Increased BMI associated with GDM leads to a state of insulin resistance additive to insulin resistance of GDM. Women with GDM are themselves very likely to develop type 2 diabetes. GDM can be recognised as a link to diabetes after pregnancy and has been proposed as a model with which to identify early metabolic defects that precede the development of diabetes in young women [4]. According to Metzger BE et al., obesity is a factor associated with increased risk of progression to diabetes within five years of GDM [5]. Intrauterine exposure of the fetus to hyperglycaemia programs the development of pancreas negatively and affects the insulin secretory function [6]. GDM pregnancies are at increased risk for perinatal morbidity and long-term obesity and glucose intolerance in offspring. With this background, the present study is aimed at assessing the relationship between GDM and pre-pregnancy BMI among pregnant women of Northern Kerala.
Materials and Methods
This hospital-based case-control study was conducted in the Out-Patient Department (OPD) and wards of Obstetrics and Gynaecology Department, Pariyaram Medical College Hospital, Pariyaram, Kannur during the period July 2015 to February 2016. Ethical clearance was obtained from the Institutional Ethical Clearance Committee for the conduct of the study. All pregnant women who attended the OPD and wards of Obstetrics and Gynaecology during the study period, who gave written informed consent were included and those with high risk pregnancies, previous history of diabetes mellitus were excluded from the study. Consecutive sampling was done with a sample size of 128 (64 cases and 64 controls), enough to detect an odds ratio of 3.7, assuming the prevalence of obesity among controls to be 14.3% with 80% power and 5% level of significance.
After obtaining the informed consent, each study subject was interviewed. Biodata, obstetric history and anthropometric measurements {height in meter (m), pre-pregnancy weight in kilograms (kg) and BMI (weight/ height2) in kg/m2} were noted from the study subjects. Pre-pregnancy BMI (BMI recorded during first antenatal visit) categorised according to WHO [7]. Values of OGTT which was done during their antenatal visits (first and second trimester) were collected and noted. OGTT estimation was done in central laboratory of Pariyaram Medical College Hospital, which was analysed by enzymatic UV test (Hexokinase method) for the quantitative determination of glucose in human serum and plasma on OLYMPUS analysers. A woman was considered to have GDM if the 2-hour plasma glucose value >140 mg/dL with the 75 gram oral glucose (DIPSI guidelines, a modified version of the WHO criterion) and rest of the women who had OGTT values within normal limits were classified as normal glucose tolerant (controls) [8]. Data obtained were tabulated and statistically analysed.
Statistical Analysis
Descriptive statistics like mean, standard deviation, frequency and Inferential statistics like independent t-test and chi-square test were used. All statistical analysis was performed using SPSS version 13.0 software. A p-value of <0.05 was considered as significant.
Results
The mean age in women with GDM was found to be 29.38±5.01 years and that in controls was found to be 26.25±4.21 years.
In the comparison of BMI, the mean±SD value in women with GDM was found to be 26.38±2.74 kg/m2 and that in controls was 22.26±1.54 kg/m2, which was found to be statistically significant [Table/Fig-1].
Comparison of BMI (kg/m2) among cases and controls.
Chi-square value (χ2) = 50.009, df=1, p≤0.001
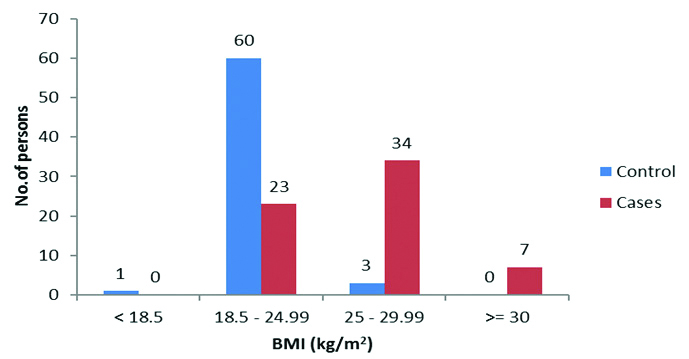
Forty one (93.2%) women with GDM had BMI ≥25 kg/m2 (overweight and obese) compared to three (6.8%) in controls, which was found to be statistically significant (χ2 value=50.01, df=1, p≤0.001). Majority of the controls (n=60) had normal BMI compared to cases (n=23) [Table/Fig-1].
Discussion
The results showed an increased risk of GDM associated with increasing BMI. Identifying such modifiable risk factors associated with GDM helps in strengthening maternal health programs by focusing on the prevention and control of obesity during the pre-pregnancy period and introducing corrective therapeutic interventions such as exercise and dietary modification.
The mean±SD value of BMI in women with GDM was found to be higher (26.38±2.74 kg/m2) compared to controls (22.26±1.54 kg/m2), which was statistically significant. Forty one (93.2%) women with GDM had BMI ≥25 kg/m2 (overweight and obese) compared to three (6.8%) in controls. In a study conducted by Bhat M et al., BMI ≥25 kg/m2 (overweight and obese) was significantly higher in cases (37.9%) compared to controls (14.3%) [9]. Seshiah V et al., observed a linear increase in the prevalence of GDM with increasing BMI in a study conducted in India [8]. A prospective longitudinal study on glucose metabolism in obese women with GDM by Catalano PM concluded that obese Gestational Diabetics had greater insulin resistance compared to normal weight women with GDM [4].
Obesity was recognised as a high risk factor necessitating early testing of glucose intolerance by the Organising Committee of the Fourth International Conference on GDM [10]. Naylor CD et al., developed a selective screening approach instead of the previously recommended universal screening for diagnosis of GDM. BMI was one of the factors on which the scoring system for categorisation of women into low, intermediate and high risk groups was based. This approach resulted in a 34.6% reduction in the number of screening tests performed without any decrease in the detection rate of GDM [11]. The American Diabetes Association (ADA) now recommends selective screening for GDM and obesity is a high risk factor [12].
Obesity is an important risk factor in the development of GDM. Obesity itself causes some degree of insulin resistance. Increased BMI associated with GDM leads to a state of insulin resistance additive to insulin resistance of GDM. Of all factors associated with GDM, obesity is a modifiable factor to a certain extent. Several mechanisms have been suggested for the increased insulin resistance in obese women with GDM. Obesity mediates a systemic inflammatory response in our body which includes insulin resistance and glucose dysregulation [9]. Plasma Free Fatty Acids (FFA) resulting from dietary fat supply and increased lipolysis in fat tissue may directly induce insulin resistance or could be channelled preferentially into triglycerides. Increased FFA uptake or lipolysis of intramyocellular lipid can inhibit insulin action via decreased insulin receptor substrate (IRS)-1 phoshorylation. Overweight and obesity has been increasing in incidence among the reproductive age group women over the past few decades in the developing countries. Catalano PM observed down regulation of IRS-1 and PPAR-γ in obese gestational diabetics more than that in non-obese GDM [4]. According to Willer KA et al., increased intramyocellular lipid concentration was found to occur in women with previous GDM and is considered as a parameter of insulin resistance that predicts type 2 diabetes [13]. Insulin resistance is much more affected by the overweight and obese women compared with lean or average weight women.
Limitation(s)
Being a hospital-based case control study, it could have been biased to a certain extent. Further studies including larger samples will substantiate our study and the results can be generalised.
Conclusion(s)
The GDM was found to be significantly associated with pre-pregnancy BMI. Appropriate interventions and risk factor modifications are recommended to prevent GDM and its complications. This study also suggests that we should pay more attention to the pre-pregnancy BMI to prevent long-term health implication of GDM on maternal and fetal health.
Author Declaration:
Financial or Other Competing Interests: None
Was Ethics Committee Approval obtained for this study? Yes
Was informed consent obtained from the subjects involved in the study? Yes
For any images presented appropriate consent has been obtained from the subjects. Yes
Plagiarism Checking Methods: [Jain H et al.]
Plagiarism X-checker: Apr 09, 2020
Manual Googling: Apr 14, 2020
iThenticate Software: Aug 22, 2020 (14%)
[1]. Ingrid O, Aspects of Gestational Diabetes: Screening system, maternal and fetal complications [Online thesis] 2003 Uppsala, SwedenTryck&Medier, Universitetstryckeriet[cited 2014, Dec 30]. Available from: http://www.divaportal.org/smash/get/diva2:162290/FULLTEXT01.pdf [Google Scholar]
[2]. Raja MW, A study to estimate the prevalence of gestational diabetes mellites in an urban block of Kashmir valley (North India)Int J Med Sci Public Health 2014 3(2):191-95.10.5455/ijmsph.2013.211120131 [Google Scholar] [CrossRef]
[3]. Sreekanthan K, Belicita A, Rajendran K, Vijayakumar A, Prevalence of gestational diabetes mellitus in a medical college in south India: A pilot studyIndian Journal of Clinical Practice 2014 25(4):342-47. [Google Scholar]
[4]. Catalano PM, Obesity, insulin resistance, and pregnancy outcome. Focus Review on ObesityReproduction 2010 140:365-71.10.1530/REP-10-008820457594 [Google Scholar] [CrossRef] [PubMed]
[5]. Metzger BE, Lowe LP, Dyer AR, Trimble ER, Chaovarindr U, Coustan DR, HAPO Study Cooperative Research Group: Hyperglycemia and adverse pregnancy outcomesN Engl J Med 2008 358:1991-2002.10.1056/NEJMoa070794318463375 [Google Scholar] [CrossRef] [PubMed]
[6]. Chu SY, Maternal obesity and risk of gestational diabetes mellitusDiabetes Care 2007 30(8):2070-76.10.2337/dc06-2559a17416786 [Google Scholar] [CrossRef] [PubMed]
[7]. World Health Organization. Measuring obesity: Classification and description of anthropometric data. Copenhagen. WHO 1989 [Google Scholar]
[8]. Seshiah V, Balaji V, Balaji MS, Sekar A, Sanjeevi CB, Green A, One step procedure for screening and diagnosis of gestational diabetes mellitusJ Obstet Gynecol 2005 55:525-29. [Google Scholar]
[9]. Bhat M, Ramesha KN, Sarma SP, Menon S, Sowmini CV, Kumar GS, Determinants of gestational diabetes mellitus: A case control study in a district tertiary care hospital in south IndiaInt J Diabetes Dev Ctries 2010 30(2):91-96.10.4103/0973-3930.62599 [Google Scholar] [CrossRef]
[10]. Oats JJ, Fourth International Workshop-Conference on Gestational Diabetes Mellitus. Overview and commentary on first sessionDiabetes Care 1998 21(Suppl 2):B58-B59. [Google Scholar]
[11]. Naylor CD, Sermer M, Chen E, Sykora K, Cesarean delivery in relation to birth weight and gestational glucose tolerance: Pathophysiology or practice styleJAMA 1996 275:1165-70.10.1001/jama.1996.035303900310308609683 [Google Scholar] [CrossRef] [PubMed]
[12]. American Diabetes Association Diabetes Care 2004 27(suppl 1):s88-s90.10.2337/diacare.27.2007.S8814693936 [Google Scholar] [CrossRef] [PubMed]
[13]. Willer KA, Krssak M, Winser C, Increased intramyocellular lipid concentration identifies impaired glucose metabolism in women with previous gestational diabetesDiabetes 2003 52:244-51.10.2337/diabetes.52.2.24412540593 [Google Scholar] [CrossRef] [PubMed]