Lichen Planus (LP) is an idiopathic, inflammatory and chronic cutaneous disease. While the systemic inflammatory nature of other autoimmune cutaneous diseases like psoriasis and alopecia areata has been studied [1,2], the pro-inflammatory nature of LP is often under-recognised. Several cytokines such as TNF-α, IL-2, and IL-6 have been implicated in the systemic inflammation in these patients [3]. Hence, it is highly likely that LP is associated with lipid derangement and atherosclerosis which will lead to increased CV risk.
Lipid profile is known to be a significant predictor of many abnormalities such as dyslipidemia, hypertension, atherosclerotic vasculature and CV diseases. The newly-addressed lipid profile: non-High Density Cholesterol (HDL-C), Total Cholesterol (TC)/ HDL-C, TG/HDL-C, and Low Density Cholesterol (LDL-C)/HDL-C ratios are proposed to be more helpful than the ordinary ones in the estimation of risk of CV diseases [4]. AIP is a logarithmically transformed ratio of molar concentrations of Triglycerides (TG) to HDL-cholesterol. CIMT is defined as the area of tissue from the lumina-intima interface to the media-adventitia interface of common carotid artery [5]. Increased CIMT may be regarded as a valid marker of generalised atherosclerosis because it is strongly associated with atherosclerosis in other parts of the arterial system of the body [6].
Hence, this study was aimed to evaluate the risk of CV disease in LP patients by studying lipid profile, AIP and CIMT and compare them with those of age and sex matched controls.
Materials and Methods
A comparative cross-sectional study was done on LP patients who attended out-patient clinic of Department of Dermatology, Rajiv Gandhi Government General Hospital, Chennai during the period between December 2019 and March 2020. The Helsinki guidelines were followed duly during the study. None of the patients had to pay for Ultrasound Sonography test (USG) examination and blood analyses expenditure.
Fifty patients with LP aged >18 years were selected as patient group. Fifty age, gender and BMI matched persons selected amongst companions of patients, patients with cosmetic problems who attended the dermatology clinic without any known dermatologic disease were selected as controls. Informed consent was obtained from them. The diagnosis of LP was based on clinical findings and was confirmed by biopsy.
The Inclusion criteria: Were the presence of LP affecting the skin or mucosa confirmed by biopsy in patients aged >18 years and the participants who have signed an informed consent before participation.
The Exclusion criteria: Were as follows: Patients aged <18 years of age, patients with metabolic syndrome and any history of drug intake (steroids, drugs which could cause lichenoid drug eruption) or history of disease (diabetes, hypertension, liver, renal, thyroid, patients with other inflammatory skin diseases) which could alter various parameters studied and patients not giving consent for the study and follow-up. It is of significance to note that metabolic syndrome was an exclusion criterion in subject selection. Many previous studies that have been done on dyslipidemia and CV risk in LP patients have not excluded patients with known metabolic syndrome [7-12] which might have acted as a confounding variable.
Methodology
History regarding disease duration, family history and personal history of patients were recorded. Dermatological and CV examinations of both groups were performed, heights and weights were measured and BMI were calculated by Quetelet index. Systolic and Diastolic blood pressure were measured after ten minutes rest.
Sample collection and biochemical analysis
Five milliliters of venous blood was withdrawn, under aseptic conditions, after 12 hours-fast and the following parameters were measured as follows:
Total Cholesterol (TC) by esterase enzymatic method.
Triglyceride (TG) by glycerol phosphate oxidase method.
High Density Lipoprotein (HDL-C) by precipitation method.
Low Density Lipoprotein (LDL-C) by Friedewald’s formula [13].
Estimation of AIP: Log (TGs/HDL-C)
Dyslipidemia was diagnosed when TGs >150 mg/dL and/or TC >200 mg/dL and/or LDL-C >130 mg/dL.
Cardiovascular (CV) risk according to AIP is shown in [Table/Fig-1] [14].
Cardiovascular risk according to AIP.
| AIP | CV risk |
|---|
| -0.3-0.1 | Low |
| 0.1-0.24 | Medium |
| >0.24 | High |
AIP: Atherogenic Index of Plasma
Ultrasound measurement of the mean intima-media wall thickness of common carotid artery
An ultrasound specialist scanned the right and left common carotid artery. Patients were lying in supine position during examination, and common carotid arteries were scanned longitudinally. CIMT was measured with 8 MHz scanning frequency of duplex ultrasound system with B-mode, pulsed Doppler mode and colour mode [15]. The right and left common carotid arteries were scanned 3 cm before the carotid bifurcation [16]. Same examiner conducted all the examinations and measurements to exclude examiner bias. Average of the values was considered as the final value. Plaque was assumed as a localised thickening >1.2 mm that did not uniformly involve the whole artery. A CIMT value >0.80 mm was considered as an index of subclinical atherosclerosis [17].
Statistical Analysis
Data was analysed using Statistical Package for the Social Sciences (SPSS) version 16.0 software. Variables were compared using Independent t-test, chi-square test and Fischer’s-exact test wherever applicable. Values were expressed in mean and proportion. Correlation between parameters was found using Pearson’s correlation coefficient. Results were considered statistically significant, if p-value was <0.05.
Results
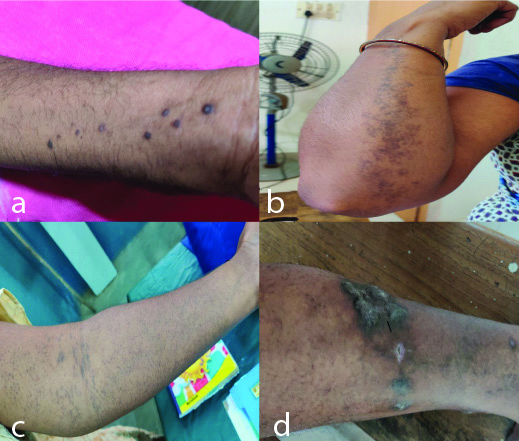
A total of 50 patients with LP and 50 healthy control subjects matched for age, gender and BMI were recruited in this study. The mean age was 43.8 (SD 12.13) years. There were 32 females and 18 males in both the groups. The average duration of disease was 3.7 years. The frequency and percentage of subtypes of LP among cases has been shown in [Table/Fig-2]. The most common subtype found in this study was classical type followed by hypertrophic type. Clinical pictures of cases have been shown in [Table/Fig-3].
Relative frequency and percentage of subtype of Lichen Planus (LP).
| Type | Frequency | Percentage (%) |
|---|
| Classical LP | 27 | 54.0 |
| Hypertrophic LP | 15 | 30.0 |
| Oral LP | 3 | 6.0 |
| Linear LP | 2 | 4.0 |
| Actinic LP | 1 | 2.0 |
| Lichen planus pigmentosus | 1 | 2.0 |
| Follicular LP | 1 | 2.0 |
AIP: Atherogenic Index of Plasma
Showing subtypes of LP: a) Classical LP; b) Linear LP; c) Follicular LP; d) Hypertrophic LP.
Comparison between patients and controls with regard to CIMT, dyslipidemia and AIP
Patients had significantly greater mean CIMT than controls with p-value <0.001 [Table/Fig-4]. A total of 14 cases (28%) and 1 control (2%) had subclinical atherosclerosis (CIMT >0.80 mm) with statistical significance of p-value <0.001 [Table/Fig-5]. There was a positive correlation between age of patients and CIMT with Pearson’s correlation of 0.474 and p-value <0.001 [Table/Fig-6].
Mean CIMT, TC, TGL, HDL, LDL and mean AIP of patients with lichen planus and healthy controls.
| Variables | | Cases | Controls | p-value |
|---|
| CIMT | Mean±SD | 0.704±0.195 | 0.526±0.076 | <0.001 |
| Range | 0.40-1.40 | 0.40-0.80 |
| T. Cholestrol (mg/dL) | Mean±SD | 196.68±37.885 | 0160.66±27.102 | 0.008 |
| TGL (mg/dL) | Mean±SD | 141.52±54.207 | 122.16±27.768 | 0.027 |
| HDL (mg/dL) | Mean±SD | 47.10±11.589 | 48.64±6.203 | 0.400 |
| LDL (mg/dL) | Mean±SD | 123.02±34.810 | 89.76±26.237 | <0.001 |
| AIP | Mean±SD | 0.100±0.226 | 0.032±0.126 | <0.001 |
| Range | (-0.317- 0.592) | (-0.239-0.404) |
CIMT: Carotid artery intima-medial wall thickness; TGL: Triglyceride; HDL: High density cholesterol; LDL: Low density cholesterol; AIP: Atherogenic index of plasma; SD: Standard deviation; p-value <0.05 significant
Prevalence of subclinical atherosclerosis (CIMT >0.80 mm) in cases and controls.
| CIMT | Chi-square value | p-value |
|---|
| <0.80 mm | >0.80 mm |
|---|
| Cases (n=50) | 36 (72%) | 14 (28%) | 13.255 | <0.001 |
| Control (n=50) | 49 (98%) | 1 (2%) |
| Total | 85 | 15 |
CIMT: Carotid artery intima-medial wall thickness; p-value <0.05 significant
Pearson Correlation betweem: age and CIMT, age and dyslipidemia, CIMT and AIP, CIMT and dyslipidemia.
| Cases | Pearson correlation (r-value) | p-value |
|---|
| Age and CIMT | 0.474 | 0.001 |
| AIP and dyslipidaemia | 0.804 | <0.001 |
| CIMT and AIP | 0.116 | 0.421 |
| CIMT and dyslipidaemia | 0.140 | 0.332 |
The mean of TC, TG, HDL-C and LDL-C were summarised in [Table/Fig-4]. Patients had significantly higher serum TG, TC, LDL compared to controls. The mean HDL was lower in patients than in controls, however statistical significance was not observed. Highly significant difference was detected between LP patients and healthy controls regarding dyslipidemia (p<0.001) [Table/Fig-7]. A total of 21 patients (42%) and only 5 controls (10%) were found to have dyslipidemia. There was no significant difference between dyslipidemic and nondyslipidemic LP patients regarding age, gender and disease duration (p>0.05).
Prevalence of dyslipidemia in cases versus controls.
| Dyslipidemia | Chi-square value | p-value |
|---|
| Normal | Dyslipidemia |
|---|
| Cases (n=50) | 29 (58%) | 21 (42%) | 13.306 | <0.001 |
| Control (n=50) | 45 (90%) | 5 (10%) |
| Total | 74 | 26 |
42% of cases and 10% of controls have dyslipidemia. p-value<0.001
AIP was significantly elevated in patient group. The mean AIP was 0.1 (SD 0.226) in patients group compared to 0.032 (SD 0.126) in controls (p<0.001) [Table/Fig-4]. Significant difference was found between both groups regarding AIP and CV risk categories. A 36% of LP patients and only 8% of controls belonged to high CV risk while 80% of controls and only 48% of LP patients belonged to low CV risk category (p<0.001) [Table/Fig-8]. Among the patients, there was no significant correlation between AIP and age, sex of patients and duration of the disease. Among the patient group, it was observed that CV risk increased with increasing age. In this study, LP patients have higher CV risk at younger mean age than controls although without statistical significance.
Comparison between case and control groups regarding atherogenic index of plasma.
| Variables | AIP | Chi-square | p-value |
|---|
| High | Intermediate | Low |
|---|
| Cases (n=50) | 18 (36%) | 8 (16%) | 24 (48%) | 13.195 | <0.001 |
| Control (n=50) | 4 (8%) | 6 (12%) | 40 (80%) |
| Total | 22 | 14 | 64 |
AIP: Atherogenic index of plasma
Statistically significant association was identified between AIP and dyslipidemia [Table/Fig-9]. LP patients with dyslipidemia had more CV risk than LP patients without dyslipidemia.
Comparison of cardiovascular risk according to AIP between dyslipidemic and normolipemic LP patients.
| Cases | AIP | p-value |
|---|
| High | Intermediate | Low |
|---|
| Normal | 1 (3.4%) | 4 (13.8%) | 24 (82.8) | <0.001 |
| With dyslipidemia | 17 (81%) | 4 (19%) | 0 |
| Total | 18 (36%) | 8 (16%) | 24 (48%) |
AIP: Atherogenic index of plasma
Discussion
In this study, LP was found to be associated with subclinical atherosclerosis, dyslipidemia, increased AIP and hence high CV risk.
LP is an autoimmune and inflammatory papulosquamous disorder of the skin, mucous membranes and appendages. Cutaneous LP is characterised by violaceous papules and plaques with intense itching causing cosmetic and psychological discomfort to the patients. Like alopecia areata and psoriasis, it is highly likely that LP is also associated with systemic inflammation. Although the pathogenesis of LP remains uncertain, T-cell mediated chronic inflammation against epidermal basal cells is considered to play a key role. There is up regulation of ICAM-1 (Intercellular Adhesion Molecule-1) and Th-1(T-helper-1) cell activity leading to increase in cytokines like IL-1, Tumour necrosis factor-alpha, IL-22, IL-4, IL-6, IL-8 and IL-17 [18-22]. Many of these cytokines have been implicated in various steps of atherosclerosis such as alteration of endothelial cells of vessel wall, recruitment, adherence and migration of lymphocytes and monocytes into inflamed vessel wall, plaque formation and adverse outcomes like plaque rupture and thrombus formation [23,24]. Chronic inflammation and production of Reactive Oxygen Species (ROS) cause lipid peroxidation, derangenment of lipid profile leading to LDL oxidation and fatty streak formation which are important steps in atherosclerosis [25,26].
The first study on dyslipidemia in LP was done as a large database study by Dreiher J et al., in 2009 in Israel [8]. Later on many studies have been done on dyslipidemia, oxidative stress [27], serum levels of homocysteine, fibrinogen and high-sensitive C-Reactive Protein (hs-CRP) [11] Neutrophil/Lymphocyte (N/L) ratio [18] and Mean Platelet Volume (MPV) [28] in LP patients. These factors which are considered as systemic inflammatory markers were elevated in LP patients. In a study on oral LP patients, it was proved that pro-inflammatory cytokines (IL-1, TNF, IL-2, IL-6 and IL-8) were increased in unstimulated saliva and oral fluids [29]. Hence, it was proposed from various previous studies that keratinocytes release many pro-inflammatory cytokines during the lymphocytotoxic process leading to systemic inflammation and free radical damage.
There are few studies about CV risk in LP patients; very few have been done in Indian population [7-12]. In this study, radiological and biochemical investigations were done in LP patients and controls to identify CV risk. Dyslipidemia is a primary, major, established and independent risk factor for coronary artery disease [30,31]. AIP is a logarithmically transformed ratio of molar concentrations of TGL to HDL-cholesterol [14]. AIP adds more predictive value than the individual lipids, and/or TC/HDL-C ratio, as a marker of lipoprotein particle size [14]. This ratio accurately reflects the presence of atherogenic small LDL and HDL particles and is considered as a sensitive predictor of coronary atherosclerosis and CV risk [32]. Dawoud NM and Bakry OA proved that AIP can be a marker of undetectable dyslipidemia in LP patients [33]. CIMT has been regarded as the ideal choice for assessing subclinical atherosclerosis in clinical practice [5].
In the present study, LP was found to be associated with subclinical atherosclerosis, dyslipidemia and increased AIP. Positive correlation was observed between age and CIMT and between dyslipidemia and AIP in LP patients [Table/Fig-6]. Correlation was not observed between CIMT and dyslipidemia/AIP [Table/Fig-6]. There were also a proportion of LP patients with high and intermediate CV risk according to AIP but with normal lipid profile [Table/Fig-9]. Hence, it can be interpreted that CIMT, dyslipidemia/AIP can be independent of each other, since chronic inflammation in LP causes both dyslipidemia and endothelial damage.
To the best of the authors’ knowledge, the three parameters- CIMT, lipid profile and AIP were not studied together in LP patients. There are very few studies done on CV risk in LP patients in Indian population. Considering the fact that CV diseases are the leading cause of mortality in India [34], this study on CV risk in a dermatological disease (whose systemic inflammatory nature is under-recognised) in Indian population with the use of both biochemical and radiological investigations in clinically asymptomatic individuals is novel, significant and credible.
Limitation(s)
The severity of LP subtypes in this study varied from limited to generalised involvement, from early stage of limited inflammation to hypertrophic and erosive stage and from reticular nonerosive type to ulcerative mucosal involvement. The analysis of the association between different subtypes and severity of LP with parameters of this study was not performed due to the small sample size in each subtype. Statistically significant correlation between CIMT and dyslipidemia/AIP was not observed. Intra group comparisons for dyslipidemia and nondyslipidemic LP patients regarding age, gender and disease duration were not statistically significant due to the small sample size. The better among the three parameters - dyslipidemia, AIP and CIMT and their ranking as a marker of CV risk could not be identified in this study.
Conclusion(s)
In this study, LP was associated with subclinical atherosclerosis, dyslipidemia, high AIP and hence high CV risk. This was in concurrence with previous studies done about systemic inflammation and CV risk in LP. Lipid profile, AIP and CIMT can serve as the tools of risk assessment in asymptomatic LP patients. Educating the patient about such a risk will help them to make lifestyle modifications to decrease the CV risk and morbidity. It is of utmost importance for the dermatologists to be aware of the systemic nature of skin diseases and insist upon their primary and secondary prevention to patients.
AIP: Atherogenic Index of Plasma
CIMT: Carotid artery intima-medial wall thickness; TGL: Triglyceride; HDL: High density cholesterol; LDL: Low density cholesterol; AIP: Atherogenic index of plasma; SD: Standard deviation; p-value <0.05 significant
CIMT: Carotid artery intima-medial wall thickness; p-value <0.05 significant
42% of cases and 10% of controls have dyslipidemia. p-value<0.001
AIP: Atherogenic index of plasma
AIP: Atherogenic index of plasma