It has long been believed that a surgical wound in the presence of a suture material has a higher risk of infection [1,2]. While around an inoculum of 100000 staphylococci were required to cause an infection in a surgical wound, even a mere 100 organisms can cause an infection in a wound in the presence of a suture [3]. This risk of the sutured wound to get infected also depends on the type of suture material used [4].
The materials for skin closure include sutures, staples, tapes and tissue adhesives. There exist approximately 5269 types of sutures [5]. Nylon is a synthetic, monofilament, non-absorbable suture with a low tissue reactivity and high tensile strength. It is most commonly used sutures for skin closure [5]. Polypropylene is also a synthetic, monofilament, non-absorbable suture with a low tissue reactivity. The tensile strength is slightly more than nylon. It has an extremely smooth surface which compromises the knot security and must be compensated with extra throws. It can pass through the tissues with minimum friction keeping the tissue response to a minimum. Its plasticity accommodates for oedema in the wound [6].
Recently the use of surgical staples have become common in many surgical specialties [7]. They have the maximum tensile strength with a minimal tissue reactivity. Studies have shown that staples show more resistance than sutures to infection from a staphylococcal inoculum [3,8,9]. There are limited studies investigating the presence and the type of microbial colonisation with respect to different skin closure techniques, especially metal staples [10,11].
The primary need of this study was to assess the microbial colonisation in association with prolene, nylon suture materials and metal staples when in contact with the skin, in the postoperative period and thus determine if there exists a significant difference in these three materials which are used commonly and often, interchangeably.
Materials and Methods
This was a hospital-based cross-sectional study performed over a duration of 20 months from a period of June 2014 to January 2016. The study was conducted on a group of subjects visiting in-patient Department of Oral and Maxillofacial Surgery of AB Shetty Memorial Institute of Dental Sciences, Mangalore, NITTE Meenakshi Institute of Craniofacial Surgery and Justice KS Hegde Charitable Hospital, undergoing craniofacial, neurosurgical and oncologic procedures of the head and neck. Institutional Ethical Clearance was obtained (Ethical clearance certificate No: ABSM/EC/47/2013). Informed consent to participate in the study was obtained from each subject.
Inclusion criteria: Patients undergoing craniofacial, neurosurgical and oncologic procedures in the head and neck area were included in the study.
Exclusion criteria: Patients on long term steroid therapy, patients with severe systemic illness, immunocompromised patients, and patients allergic to nylon, prolene or stainless steel materials were excluded from the study. Also, patients in which the site of surgery had a pre-existing infection were excluded.
Methodology
A total of 60 patients were selected for this pilot study and were further categorised into three groups:
Group I: Patients in whom surgical closure was performed with prolene (PROLENE, Ethicon, Johnson & Johnson Ltd., India).
Group II: Patients in whom surgical closure was performed with Nylon (ETHILON, Ethicon, Johnson & Johnson Ltd., India).
Group III: Patients in whom surgical closure was performed with surgical metal staples (Visistat 35 W, TFX Ltd., UK).
The parameters studied were as follows:
1. Microbial Colonisation
The subjects underwent various surgical procedures of head and neck area such as craniofacial, neurosurgical and oncologic procedures (the patients were not given any chemotherapy surgery and had normal white blood cell count). The surgical wound was maintained during the postoperative period by regular dressings.
The sutures/staples were removed on 8th to 14th postoperative day. The removed suture fragment or the staple were placed in sterile containers containing normal saline at room temperature and carried to the laboratory where they were inoculated immediately, aerobically in suitable culture media according to the standard methodology [12]. The cultures were done on Blood Agar, Mutans Sanguis Agar, McConkey agar and Sabouraud’s Dextrose Agar. The further identification was then done using Gram’s stain and biochemical reactions. Mutans sanguis agar was used to identify Streptococcus mutans commonly found in saliva. Head and neck area have a small chance of getting contaminated with saliva due to the proximity of oral cavity. Authors wanted to rule out any such contamination. None of the samples were found to be contaminated with Streptococcus mutans. Sabouraud’s dextrose agar was used to identify fungi such as Candida which is commonly found in skin wound infections.
2. Complications
Patient was assessed for the presence or absence of complications such as dehiscence, gaping, and necrosis at the time of the removal of sutures or staples i.e., 8-14 days.
Statistical Analysis
The data was analysed using Statistical Package for the Social Sciences (SPSS) software v18. The results were derived using repeated measures ANOVA, Chi-square test and Fisher’s-exact test. The p-value ≤0.05 was considered as statistically significant.
Results
The mean age of the patients in Group I was found to be 16.71 years, group II was 47.80 years and Group III was 54.5 years [Table/Fig-1]. The gender distribution among the three groups was 11 males and 9 females in Group I, 10 males and 10 females in Group II; and 13 males and 7 females in Group III [Table/Fig-2].
Age distribution among the three groups.
| Age (years) |
|---|
| Group | N | Mean | Std. deviation | Minimum | Maximum |
|---|
| I | 20 | 16.71 | 20.78 | 0.42 | 64.00 |
| II | 20 | 47.80 | 15.78 | 11.00 | 67.00 |
| III | 20 | 54.50 | 11.11 | 24.00 | 70.00 |
Gender distribution among the three groups.
| Gender | Group | Total |
|---|
| I | II | III |
|---|
| Male | 11 (55.0%) | 10 (50.0%) | 13 (65.0%) | 34 (56.7%) |
| Female | 9 (45.0%) | 10 (50.0%) | 7 (35.0%) | 26 (43.3%) |
| Total | 20 (100.0%) | 20 (100.0%) | 20 (100.0%) | 60 (100.0%) |
| Chi-square value -0.95 (2), p=0.62 (NS) |
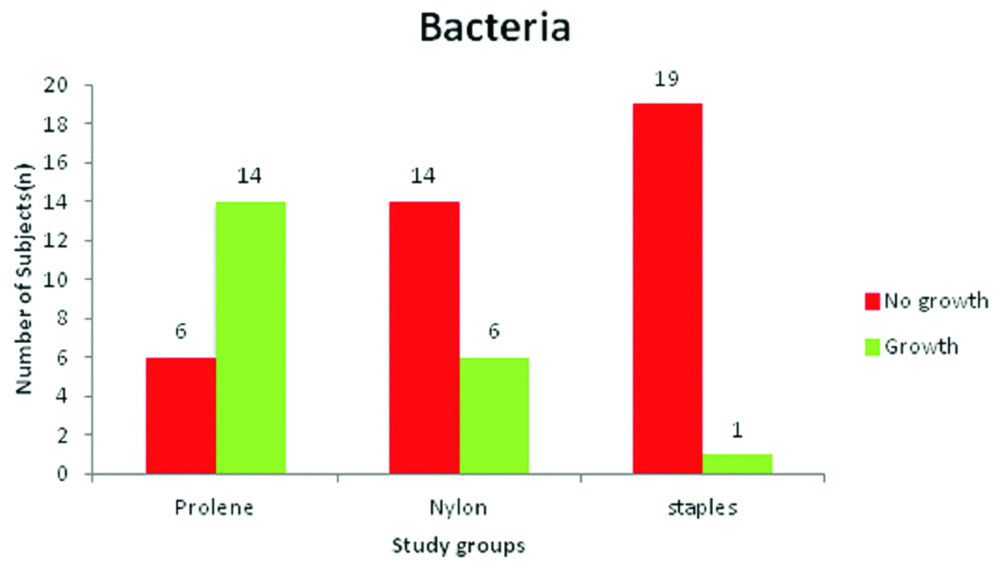
A total of 70% of the patients from group I were found to have bacterial growth on microbial colonisation, whereas 30% did not show any growth. In group II, 30% of the patients demonstrated bacterial growth whereas the rest of the 70% showed no growth. In group III 95% of the patients did not show any bacterial growth on culture media and only 5% showed positive bacterial growth. All of these differences were statistically significant (p<0.001) [Table/Fig-3].
Graph comparing the presence of bacterial colonisation in the three groups.
Chi square value -18.90 (2), p<0.001
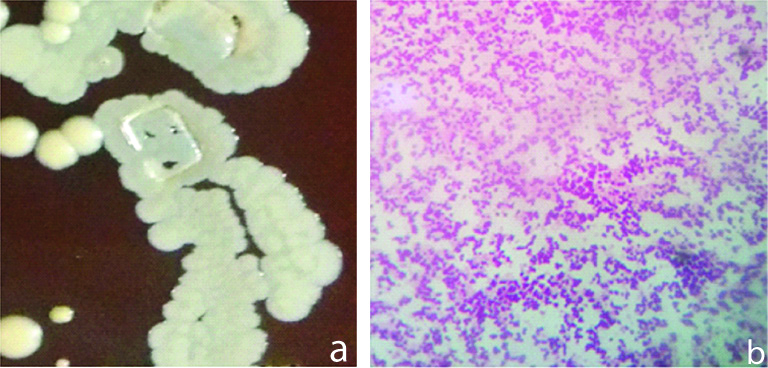
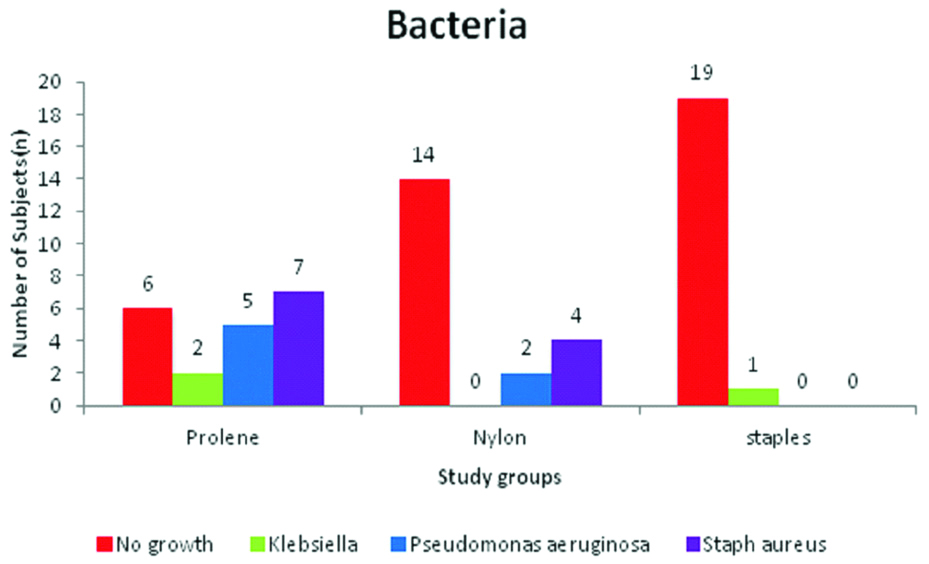
On blood agar, beta haemolytic colonies were found. On MacConkey agar, lactose fermenting small pink colonies were found. Biochemical tests such as carbohydrate metabolism, oxidase and catalase tests were performed to confirm the organisms. In Group I, the most common bacteria on culture was Staphylococcus aureus (35%) [Table/Fig-4a,b] followed by Pseudomonas aeruginosa (25%) and Klebsiella spp (10%).
a) Staphylococcus aureus on blood agar showing beta haemolytic colonies seen by naked eye. b) Staphylococcus gram staining seen on light microscope using oil immersion lens, appearing as gram positive cocci.
In group II, 20% of the positive cultures showed the growth of Staphylococcus aureus and 10% showed Pseudomonas aeruginosa.
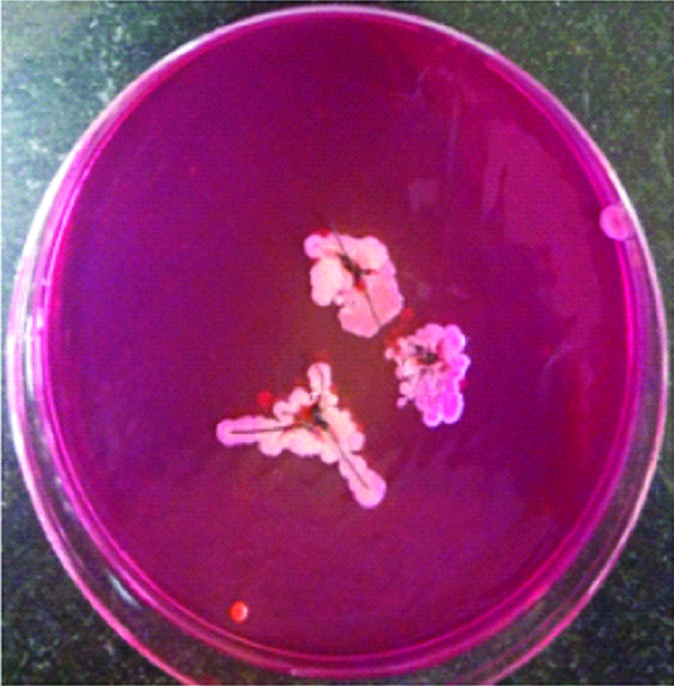
In group III, of the 5% of the samples which showed positive growth, all showed Klebsiella spp, these differences were found to be statistically significant [Table/Fig-5,6]. None of the samples in any of the groups showed fungal growth on culture media after incubation for seven days.
Klebsiella spp colonies on MacConkey agar showing small pink colonies.
Graph comparing the presence and the type of micro-organisms in the three groups.
Fisher’s exact test -21.15, p<0.001
The complications in all the three groups were categorised as either present or absent. The wound complication that was observed was gaping. Necrosis or dehiscence was not observed in any of the subjects. Gaping was present in 5% of the patients of Group I, 15% of the patients and 10% of the patients of group III. However, these differences were not statistically significant using Fisher’s-exact test (p=0.86] [Table/Fig-7].
Incidence of gaping in the three groups.
| Complication | Group | Total |
|---|
| I | II | III |
|---|
| Gaping absent | 19 (95.0%) | 17 (85.0%) | 18 (90.0%) | 54 (90.0%) |
| Gaping present | 1 (5.0%) | 3 (15.0%) | 2 (10.0%) | 6 (10.0%) |
| Total | 20 (100.0%) | 20 (100.0%) | 20 (100.0%) | 60 (100.0%) |
| Fisher’s-exact test -1.14, p=0.86 |
Discussion
The presence of sutures in a surgical wound increases the tissue’s susceptibility to infection [3,9]. Occurrence of bacteria inside a wound does not always implicate an infection. Altemeier WA and Culberston WR have suggested that the infection risk depends upon the host resistance, bacterial contamination and virulence of the organism [13]. In human volunteers, Elek SD and Conen PE have noted that an injection of 106 organisms of Staphylococcus pyogenes was required to elicit a pus-forming clinical infection [3]. When a silk suture was introduced into the same condition only 102 staphylococci were enough to form pus. The results of this study indicate that the introduction of staphylococci on a silk suture can enhance the development of infection as much as thousand fold.
The tissue-handling technique also has a significant effect on the infection rate. It is believed that factors like extremely tight sutures and the inflammatory response of the tissues due to the passage of the needle increases the risk of infection. Incidence of wound infection also depends on the physical morphology and chemical foundation of the skin closure material [1].
Bacteria are known to lower the oxygen tension in the wound and decrease the proliferation of fibroblasts. This modified local environment delays wound healing. Thus, it can be inferred that suture materials, by allowing colonisation of more number of bacteria, are responsible for a delay in the wound healing [14]. Colonisation is the initial step in the process of developing infection. It is believed that if colonising microbes are eradicated from the host by any of the modalities no more progress towards the infection will occur. If the colonisation persists, it will lead to infection [15].
Prevention of surgical site infection depends on a variety of factors which include antibiotics, asepsis, surgical technique and wound closure materials [1,16]. Awareness of affinity of various wound closure methods towards microbial colonisation is therefore essential [10]. Considering this fact, authors studied the microbial colonisation in relation to three wound closure materials to predict which of the three materials will result in greater incidence of wound infection. In the current study, prolene showed highest rate of microbial colonisation (70%), followed by nylon (30%) and staples (5%). These findings were similar to those of Edlich RF et al., who also reported a higher colonisation rate in the case of prolene compared to nylon, however the difference was not statistically significant [1]. Ritchie AJ and Rocke LG in their prospective double blinded randomised trial found no significant difference in the infection rates in wounds with staples and nylon suture material [17]. Both nylon and prolene are monofilament synthetic suture materials with a minimal reactivity in tissues. On a thorough literature search, studies comparing the infection rates of nylon and prolene suture materials were not found. Authors postulated the following possible reasons for a higher microbial colonisation in prolene compared to nylon. As mentioned earlier, strangulation of tissue by tying the sutures tight may raise the chances of wound infection [1]. Since prolene has a smooth surface along with low knot security, there can be a tendency for excessive tightening of the knot and multiple knots leading to increased susceptibility to wound infection. Edlich RF et al., conducted in vitro studies where they found the degradation products of nylon (1,6 hexanediamine and adipic acid) as efficacious antibacterial agents. When Staphylococcus aureus was in incubated with 1,6 hexanediamine and adipic acid, it was seen that the microbial count reduced markedly. They proposed that this antimicrobial property of the end products of nylon suture may kill some bacteria and reduce the reaction tissues to the suture [1]. The results were also in accordance with Katz S et al., who showed that the bacteria show a variable affinity towards different variety of sutures [18]. They also proved that the antimicrobial property was good in case of nylon. According to their study, the bacteria adhered to nylon to the smallest extent. Similarly Rothenburger S et al., have also proved that wound infection depends on the structure and the type of suture material [19].
In the present study, the most common colonising bacteria was Staphylococcus aureus followed by Pseudomonas aeuginosa and Klebsiella spp. These findings were in accordance with a study conducted by Edmiston CE et al., who found that common organisms colonising sutures include coagulase-negative staphylococci, Staphylococcus aureus, Pseudomonas aeruginosa, Peptostreptococcus spp, Escherichia coli, Staphylococcus epidermidis, Bacteroides fragilis and Serratia species [10]. Although a skin commensal, it is noteworthy that staphylococci are responsible for a number of skin infections [20,21] and may undoubtedly play a role in causing a wound infection in the presence of a suture. Gram negative organisms such as Klebsiella spp, Pseudomonas are non-commensals known to have a role in skin infections [20].
This study showed that staples showed less incidence of microbial colonisation compared to suture materials. This finding was in accordance with Carcoforo P et al., and Stillman RM et al., [22,23]. Alexander JW et al., showed lower incidence of infection with monofilament wire compared to monofilament nylon [4]. The lower infection rate in stainless steel staples could be due to low tissue reactivity demonstrated by stainless steel material [5]. Present results did not comply with that of Edlich RF et al., and Figueroa D et al., who concluded that staples were more prone to infection compared to sutures [1,24]. The present results also did not match with a meta-analysis by Smith TO et al., who demonstrated that in orthopaedic procedures, staples had a three times higher risk of infection compared to sutures [25]. However, some of the studies included in this meta-analysis used vicryl, which is a multifilament suture material known to harbour more micro-organisms compared to the monofilament ones. According to the present study, prolene was found to harbour higher microbial colonisation compared to nylon and staples. In this scenario antibiotic coated prolene may aid in better control of microbial activity. Sutures coated with triclosan have demonstrated decrease in the colonisation of coagulase-negative Staphylococcus and staph. Staphylococcus aureus and Pseudomonas spp [26-28].
Limitation(s)
Conventional culture methods were used to identify the different micro-organisms. However, use of advanced investigations such as mass spectrometry microbial identification system such as VITEK MS that uses uses Matrix Assisted Laser Desorption Ionisation Time-of-Flight (MALDI-TOF) technology, are more sensitive and help in better identification of the microbiota [29]. Future studies with inclusion of anaerobic organisms are recommended. The sample size was small and had wide age range (Group I has less mean age than Group II and III). Further prospective studies with a larger sample size of comparable age group are required.
Conclusion(s)
The findings of this study suggested that prolene was most prone to bacterial colonisation followed by nylon and staples. If feasible, surgical staples are preferable in cases which have predisposition to infection. The most common micro-organisms found were Staphylococcus aureus, Pseudomonas aeruginosa and Klebsiella spp. Also, there was no significant difference between the complication rate of nylon, prolene and surgical staples. Studies with a larger sample size are recommended to further confirm these findings.