Pulmonary Thromboembolism in COVID-19: Initial Experience from India
Shekhar Kunal1, Harnish Bhatia2, Sohan Kumar Sharma3, Shashi Mohan Sharma4, Sudhir Bhandari5
1 Senior Resident, Department of Cardiology, SMS Medical College, Jaipur, Rajasthan, India.
2 Senior Resident, Department of Cardiology, SMS Medical College, Jaipur, Rajasthan, India.
3 Associate Professor, Department of Cardiology, SMS Medical College, Jaipur, Rajasthan, India.
4 Senior Professor and Head, Department of Cardiology, SMS Medical College, Jaipur, Rajasthan, India.
5 Senior Professor, Department of Medicine, SMS Medical College, Jaipur, Rajasthan, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Sohan Kumar Sharma, SMS Medical College, Jaipur, Rajasthan, India.
E-mail: drsohansharma@yahoo.com
Pulmonary thromboembolic complications are increasingly being recognised in Coronavirus Disease 2019 (COVID-19) infections. Most of the cases of Pulmonary Embolism (PE) are often missed in presence of non-specific symptoms. The present report is about a 90-year-old COVID-19 positive male, asymptomatic on presentation, with no prior co-morbidities who developed acute onset shortness of breath along with elevated D-dimer levels four days post admission. Subsequently, a Computed Tomography Pulmonary Angiogram (CTPA) was done which revealed segmental and sub-segmental thromboembolism in upper and middle lobar branches of right pulmonary artery along with bilateral lower lobe ground-glass opacities consistent with COVID-19 pneumonia. As the patient was haemodynamically stable, he was managed conservatively on low molecular weight heparin and subsequently discharged on oral anti-coagulants. This report highlights the need for prompt evaluation of symptoms such as dyspnoea in COVID-19 patients and to rule out thromboembolic complications in them. In resource limited countries such as India with most of the COVID-19 centres having limited access to CT scans, triaging patients based on clinical suspicion and serially rising D-dimer levels may help identify those with thromboembolic complications.
Anticoagulation, D-dimer, Pneumonia
Case Report
A 90-year-old male with no prior co-morbid conditions came to medical attention due to a history of contact with a COVID positive patient. He had been physically active prior to hospitalisation and even during the course of his hospital stay. He was a non-smoker and had no prior history of any drug or alcohol intake. On presentation, patient was asymptomatic and had been admitted for institutional quarantine for 14 days which was a protocol during the initial phase of COVID-19 pandemic. General physical examination revealed an elderly male with an average built, afebrile, pulse rate of 55 beats per minute, blood pressure of 110/70 mmHg and respiratory rate of 14/min with SpO2 98% while breathing ambient air. Systemic examination was unremarkable. Routine laboratory investigations revealed haemoglobin of 14 gm% with Total Leucocyte Count of 7000/μL and an absolute lymphocyte count of 874/μL suggestive of lymphopenia. His Renal and Liver Function Tests were normal. He had tested positive for COVID-19 in his throat swab by Reverse Transcriptase Polymerase Chain Reaction (RT-PCR) testing.
Electrocardiogram (ECG) on admission was suggestive of first degree Atrio-Ventricular (AV) block, slightly prolonged QTc of 490 msec along with incomplete right bundle branch block. Cardiac troponins, CK-MB and D-dimer levels were normal (650 μg/L; age-adjusted cut-off: 900 μg/L). Chest X-ray on presentation was reported to be normal with slight elevation of the left hemi-diaphragm while two dimensional echocardiography revealed normal cardiac chamber dimension and normal biventricular systolic function. On the fourth day of the admission, patient developed sudden onset shortness of breath and pleuritic chest pain lasting for a couple of hours. Pulse oximetry revealed a SpO2 of 93% and on examination, he was haemodynamically stable. Repeat ECG was suggestive of persistent first-degree AV block and development of new onset S waves in lead I with a significant elevation in D-dimer (3000 μg/L; age-adjusted cut-off: 900 μg/L) and Fibrin Degradation Products (FDP) (9.6 μg/mL; Normal: <5 μg/mL) levels. In addition, C-Reactive Protein (CRP) was positive, serum Lactate Dehydrogenase (LDH) and ferritin levels were raised with a normal serum pro-calcitonin level. Test for various coagulation parameters and factors were normal.
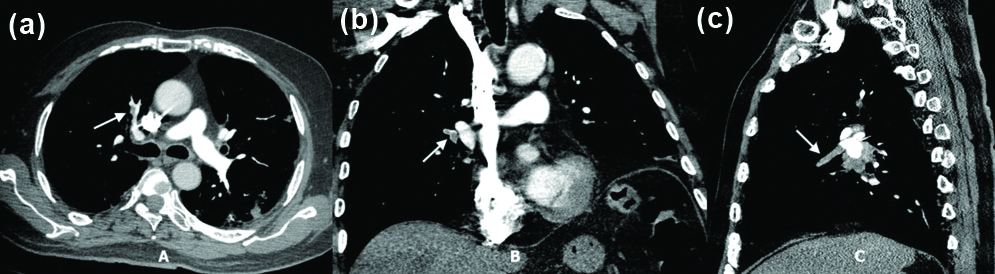
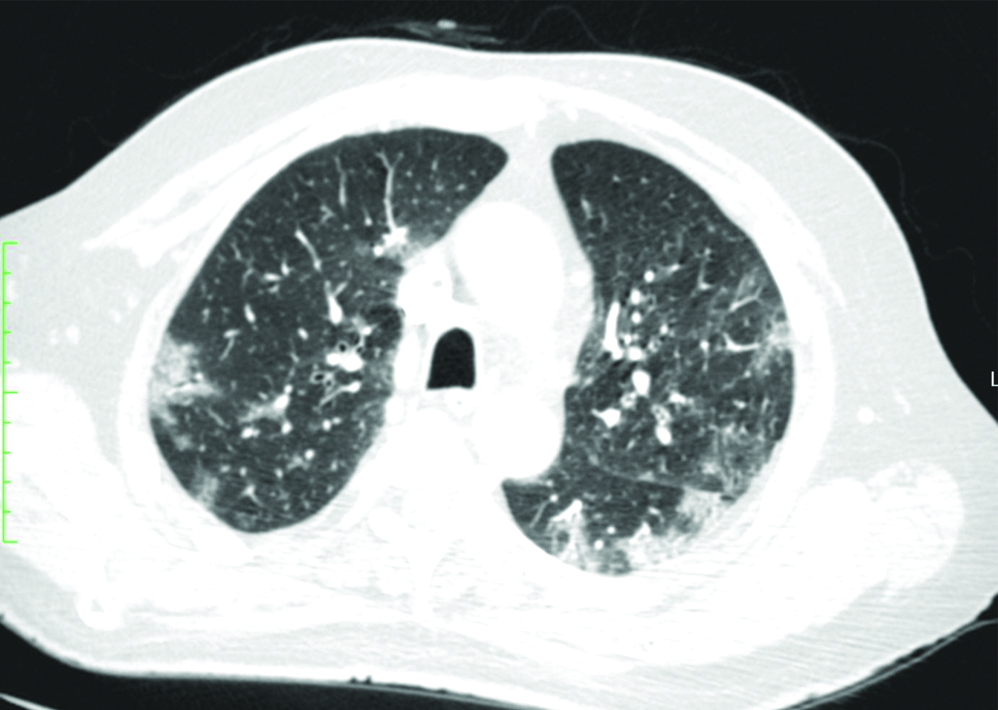
In view of a clinical suspicion of pulmonary thromboembolism based on the history of sudden onset of shortness of breath along with elevated D-dimer levels, patient underwent CTPA, which showed acute thrombosis of segmental and sub-segmental branches of upper and middle lobar branches of right pulmonary artery along with dilatation of the main pulmonary trunk [Table/Fig-1]. In addition, the high resolution CT of the chest was suggestive of multifocal areas of ground glass opacities in the bilateral lung parenchyma with a peripheral and lower lobe predominance typical of COVID-19 infection [Table/Fig-2]. Subsequently, an echocardiogram showed slight dilation of the right ventricle with mild tricuspid regurgitation {Right Ventricular Systolic Pressure (RVSP)=right atrial pressure+30}. Lower limb doppler did not reveal any evidence of deep venous thrombosis. Ultrasound abdomen evaluation was normal and screening for any malignancy was negative.
a) CT Pulmonary Angiogram (CTPA) axial section showing acute thrombus in the right upper lobe pulmonary artery (white arrow). b) CT Pulmonary Angiogram (CTPA) coronal section showing an acute thrombus in the right interlobar pulmonary artery (white arrow). c) CT Pulmonary Angiogram (CTPA) sagittal section showing an acute thrombus in the right middle lobe pulmonary artery (white arrow).
High resolution computed tomography of the chest (axial section) showing multifocal areas of ground glass opacities in bilateral lung parenchyma with a peripheral and lower lobe predominance (left > right) typical of COVID-19 infection.
In view of his stable haemodynamic status, patient was started on injection low molecular weight heparin overlapped with oral anti-coagulation. His symptoms abated in a couple of days and was discharged on oral anti-coagulants on the 11th day of admission in an asymptomatic state with a normal body temperature (37.2°C) and two negative Real Time-Polymerase Chain Reaction (RT-PCR) tests for Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2). After one month of his discharge, the patient is asymptomatic and has been continuing oral anti-coagulant therapy.
Discussion
COVID-19 originated from Wuhan, China in late December 2019 and has so far spread to more than 200 countries across the globe [1]. Even though its primarily a respiratory illness, it affects multiple organ systems which determines the course of the disease and its prognosis. It can present in a multitude of ways ranging from an asymptomatic case to a full blown severe systemic illness characterised by multisystem involvement, Acute Respiratory Distress Syndrome (ARDS), myocarditis, renal failure, coagulation abnormalities and even death [2,3]. This multisystem involvement is caused by a combination of specific host defence mechanisms leading to a cascade of inflammatory activity, “cytokine storm” and microvascular involvement with associated coagulopathy and propensity to develop thromboembolic manifestations [2]. Venous thromboembolic events are increasingly being recognised as one of the common complications of COVID-19 infection especially in severe forms of the disease [4,5]. In addition, a substantial proportion of patients develop arterial and venous thromboembolic complications which often undergo unrecognised. Initial reports from China suggested that haemostatic abnormalities are prevalent in COVID-19 patients which implied a poor prognosis [6,7]. Common laboratory findings in those studies were lymphopenia, thrombocytopenia, elevated LDH levels and other inflammatory markers such as CRP, ferritin, D-dimer and Interleukin-6 (IL-6). In a study by Tang N et al., from China, 71.4% of non-survivors and 0.6% survivors met the International Society on Thrombosis and Haemostasis criteria of disseminated intravascular coagulation during their hospital stay [6]. All these together does indicate some form of coagulopathy predisposing to thromboembolic complications.
The mechanisms underlying the coagulopathy and thromboembolic manifestations in COVID-19 infection are largely unknown. It is still not clear whether this hypercoagulable state is due to the specific effects of SARS-CoV-2 or as a result of the cytokine storm associated with systemic inflammatory response syndrome in these patients [8]. In addition, there seems to be a contributory role of antiphospholipid antibodies in severe COVID-19 patients as was evident in a recently published series from China [9]. Critically ill patients are often prone to develop thromboembolic complications as a result of venous stasis due to prolonged immobilisation. In addition, other factors promoting hypercoagulability include use of oral or intravenous glucocorticoids, immunoglobulins and other investigational therapies for COVID-19 as well as vascular endothelial damage from central venous catheterisation and/or Extra-Corporeal Membrane Oxygenator (ECMO) [2]. Pro-inflammatory cytokines leads to activation of coagulation cascade as well as endothelial injury in these patients. All of these act together as multiple contributing factors for the occurrence of Venous Thromboembolism (VTE) in critically ill COVID-19 patients [2]. Whatever the cause may be, these haemostatic abnormalities portrays a poor prognosis and untoward outcomes. Hence, timely identification and correction of those abnormalities forms an important component in management of COVID-19 positive patients. Limited data exists regarding the incidence and characteristics of thromboembolic complications in hospitalised patients with COVID-19 infection. In a series from France [10], the reported prevalence of PE in COVID-19 patients was 20.6% (22/107 successive ICU admissions). This seemed to be alarmingly high as compared to the COVID-19 negative ICU patients where its prevalence was reported to be only 6.1%. This reflected a 14.5% absolute increase in risk of PE in COVID-19 positive patients. Similarly, in an another series of 184 critically ill patients with COVID-19 pneumonia, the incidence of thrombotic complications was reported to be 31% [11]. In a study by Grillet F et al., the reported incidence of PE was 23% in severe COVID-19 cases with multivariate analysis showing a higher frequency in males and those undergoing invasive mechanical ventilation and males [12]. In an autopsy based series of 12 consecutive COVID-19 deaths, Wichmann D et al., reported deep venous thrombosis in 7/12 patients (58%) in whom VTE was not suspected before death [13]. PE was identified to be the direct cause of death in four patients only at autopsy. This study suggested that the incidence of PE could be much higher than estimated.
Dyspnoea as a presenting symptom in COVID-19 infection is often non-specific and can be attributed to pneumonia, ARDS, myocarditis, heart failure or PE in these patients [2]. Most of the COVID-19 patients diagnosed with PE are critically ill with fever, cough, chest pain and dyspnoea as the predominant symptoms. Dyspnoea as a symptom in the natural history of COVID-19 infection is often seen later in the course of the disease process occurring “between the fourth and eighth day of illness” or even later often due to the underlying lung involvement [14]. As such, in patients presenting with acute onset dyspnoea as an initial symptom in COVID-19 patients, a high probability of PE should be kept in mind. Guidelines and position statements from the radiological societies of developed countries such as European Society of Radiology and the European Society of Thoracic imaging suggest that CTPA to rule out PE should be done if there is need of supplementary oxygen in COVID-19 pneumonia patients with “limited pulmonary involvement” [15]. However, in developing countries with resource constrained setups and limited access to CT scans dedicated for COVID-19 patients, clinical evaluation coupled with rising D-dimer levels would help select the patients for CTPA to rule out PE. Management of PE in COVID-19 patients is very similar to that of COVID negative patients. As per the European Society of Cardiology guidelines [16], in patients with overt haemodynamic instability (high-risk PE) systemic fibrinolysis is indicated while most of the intermediate-risk haemodynamically stable patients can be managed with anti-coagulation and close monitoring alone. Controversy exists regarding the use of routine thromboprophylaxis in COVID-19 patients with few authors favouring use of low molecular weight heparin in patients with severe pneumonia and excessive activation of coagulation cascade. However, a recent study showed that prophylactic anticoagulation did not avoid the occurrence of PE in hospitalised patients [17]. However, the recently published CHEST guidelines on prevention, diagnosis and treatment of VTE in COVID-19 advocates for anticoagulant thromboprophylaxis in acutely ill hospitalised/critically ill patients [18].
Conclusion(s)
Thromboembolic complications are increasingly being recognised in COVID-19 and often portray a bad prognosis. A limited body of experience exists regarding the epidemiology and pathophysiology of VTE in COVID-19. The clinical diagnosis of VTE in COVID-19 poses a challenge as most of the patients with COVID-19 infection presents with dyspnoea. Hence, a high index of suspicion should be kept regarding the presence of VTE in these patients with prompt diagnosis and management. There is a need for further large scale studies documenting the prevalence as well as the impact of thromboembolism in COVID-19 patients.
Author Declaration:
Financial or Other Competing Interests: None
Was informed consent obtained from the subjects involved in the study? Yes
For any images presented appropriate consent has been obtained from the subjects. Yes
Plagiarism Checking Methods: [Jain H et al.]
Plagiarism X-checker: Jul 02, 2020
Manual Googling: Jul 09, 2020
iThenticate Software: Aug 10, 2020 (12%)
[1]. Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, China novel coronavirus investigating and research team. A novel coronavirus from patients with pneumonia in China, 2019N Engl J Med 2020 382:727-33.10.1056/NEJMoa200101731978945 [Google Scholar] [CrossRef] [PubMed]
[2]. Kunal S, Gupta K, Sharma SM, Pathak V, Mittal S, Tarke C, Cardiovascular system and COVID-19: Perspectives from a developing countryMonaldi Arch Chest Dis 2020 90:1010.4081/monaldi.2020.130532380802 [Google Scholar] [CrossRef] [PubMed]
[3]. Du RH, Liang LR, Yang CQ, Wang W, Cao TZ, Li M, Predictors of mortality for patients with COVID-19 pneumonia caused by SARS-CoV-2: A prospective cohort studyEur Respir J 2020 55:200052410.1183/13993003.00524-202032269088 [Google Scholar] [CrossRef] [PubMed]
[4]. Tal S, Spectre G, Kornowski R, Perl L, Venous thromboembolism complicated with COVID-19: What do we know so far?Acta Haematol 2020 :01-08.10.1159/00050823332396903 [Google Scholar] [CrossRef] [PubMed]
[5]. Nahum J, Morichau-Beauchant T, Daviaud F, Echegut P, Fichet J, Maillet JM, Venous thrombosis among critically ill patients with coronavirus disease 2019 (COVID-19)JAMA Netw Open 2020 3:e201047810.1001/jamanetworkopen.2020.1047832469410 [Google Scholar] [CrossRef] [PubMed]
[6]. Tang N, Li D, Wang X, Sun Z, Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumoniaJ Thromb Haemost 2020 18:844-47.10.1111/jth.1476832073213 [Google Scholar] [CrossRef] [PubMed]
[7]. Tang N, Bai H, Chen X, Gong J, Li D, Sun Z, Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathyJ Thromb Haemost 2020 18:1094-99.10.1111/jth.1481732220112 [Google Scholar] [CrossRef] [PubMed]
[8]. Bone RC, Grodzin CJ, Balk RA, Sepsis: A new hypothesis for pathogenesis of the disease processChest 1997 112:235-43.10.1378/chest.112.1.2359228382 [Google Scholar] [CrossRef] [PubMed]
[9]. Zhang Y, Zhang Y, Xiao M, Zhang S, Xia P, Cao W, Coagulopathy and antiphospholipid antibodies in patients with covid-19N Engl J Med 2020 382:e3810.1056/NEJMc200757532268022 [Google Scholar] [CrossRef] [PubMed]
[10]. Poissy J, Goutay J, Caplan M, Parmentier E, Duburcq T, Lassalle F, Lille ICU Haemostasis COVID-19 group. Pulmonary embolism in covid-19 patients: Awareness of an increased prevalenceCirculation 2020 Apr 24 [Google Scholar]
[11]. Klok FA, Kruip MJHA, van der Meer NJM, Arbous MS, Gommers DAMPJ, Kant KM, Incidence of thrombotic complications in critically ill ICU patients with COVID-19Thromb Res 2020 191:145-47.10.1016/j.thromres.2020.04.01332291094 [Google Scholar] [CrossRef] [PubMed]
[12]. Grillet F, Behr J, Calame P, Aubry S, Delabrousse E, Acute pulmonary embolism associated with COVID-19 pneumonia detected by pulmonary CT AngiographyRadiology 2020 :20154410.1148/radiol.202020154432324103 [Google Scholar] [CrossRef] [PubMed]
[13]. Wichmann D, Sperhake JP, Lütgehetmann M, Steurer S, Edler C, Heinemann A, autopsy findings and venous thromboembolism in patients with COVID-19Ann Intern Med 2020 :M20-2003.10.7326/L20-120633316197 [Google Scholar] [CrossRef] [PubMed]
[14]. Cohen PA, Hall LE, John JN, Rapoport AB, The early natural history of SARS-CoV-2 infection: Clinical observations from an Urban, Ambulatory COVID-19 ClinicMayo Clin Proc 2020 95:1124-26.10.1016/j.mayocp.2020.04.01032451119 [Google Scholar] [CrossRef] [PubMed]
[15]. Revel MP, Parkar AP, Prosch H, Silva M, Sverzellati N, Gleeson F, European Society of Radiology (ESR) and the European Society of Thoracic Imaging (ESTI). COVID-19 patients and the radiology department- advice from the European Society of Radiology (ESR) and the European Society of Thoracic Imaging (ESTI)Eur Radiol 2020 :01-07.10.1007/s00330-020-06865-y32314058 [Google Scholar] [CrossRef] [PubMed]
[16]. Konstantinides SV, Meyer G, Becattini C, Bueno H, Geersing GJ, Harjola VP, ESC Scientific Document Group2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS)Eur Heart J 2020 41:543-603.10.1093/eurheartj/ehz40531504429 [Google Scholar] [CrossRef] [PubMed]
[17]. Bompard F, Monnier H, Saab I, Tordjman M, Abdoul H, Fournier L, Pulmonary embolism in patients with Covid-19 pneumoniaEur Respir J 2020 :200136510.1183/13993003.01365-202032398297 [Google Scholar] [CrossRef] [PubMed]
[18]. Moores LK, Tritschler T, Brosnahan S, Carrier M, Collen JF, Doerschug K, Prevention, diagnosis, and treatment of VTE in patients with COVID-19: CHEST Guideline and Expert Panel ReportChest 2020 S0012-3692(20)31625-1 [Google Scholar]