Introduction
Vitamin K Antagonists (VKAs) have been in use for more than 50 years. They have remained as mainstay therapy in the prevention of thromboembolic events in atrial fibrillation, mechanical heart valves and venous thromboembolism. Despite many years of clinical experience with VKAs, the quality of anticoagulation achieved in routine clinical practice is suboptimal.
Aim
To study the effects of structured Anticoagulation Clinic (ACC) interventions on patient centred outcomes in subjects taking VKAs.
Materials and Methods
A retrospective study was conducted among patients taking VKAs enrolled in ACC. A total of 169 patients receiving VKAs for at least six months with 4 INR (International Normalised Ratio) values and completed 12 months of follow-up were analysed. Anticoagulation related quality measures like Time in the Therapeutic Range (TTR), Percentage of International Normalised Ratios in the therapeutic Range (PINRR) and clinical outcomes like stroke, systemic embolic events and bleeding was analysed at the time of enrolment and compared with those during ACC care.
Results
Among 352 patients enrolled in ACC, 169 patients were eligible for analysis. The mean age of the study population was 55.62±13.77 years. Atrial fibrillation (59%) was the most common indication for VKA therapy. Hypertension (66.3%) was the most common co-morbidity. Mean TTRs were significantly higher in the ACC care when compared with the pre-ACC care at 12 months follow-up (77.58±8.85% vs 51.01±16.7%, p<0.0001). There was a significant improvement in TTRs as early as three months of ACC intervention (73.18±13.56%). At the time of enrolment, 21.9% of patients had individual TTRs (i-TTR) >70% which increased to 70.4% at 12 months of follow-up. INR testing was done more frequently in ACC care. Adverse clinical events were higher in pre-ACC care than ACC care (4.7% vs 2.4%, p>0.05). Major bleeding and thromboembolic events were higher in pre-ACC care than ACC care (1.8% vs. 0.6% and 2.4% vs. 0.6% respectively).
Conclusion
ACC services helps in achieving better quality of anticoagulation control as measured by time in therapeutic range translating into better clinical outcomes.
Introduction
Vitamin K antagonists (VKAs) are effective in reducing thromboembolic events in patients with atrial fibrillation, mechanical heart valves and venous thromboembolism [1,2]. In India, VKAs are the most commonly prescribed anticoagulants because of several years of clinical experience, data supporting its efficacy and the prohibitive cost of Non-Vitamin K Oral Anticoagulants (NOACS) [3]. However, there are challenges to use VKAs for instance, the need for frequent monitoring of INR, variable dose-response, possible interactions (diet and drugs), and risk of major bleeding which can lead to significant morbidity and mortality [4]. It is reported that most patients on VKAs spend a large proportion of their time with an INR value outside their target range [5]. Fear of bleeding complication and the need for frequent monitoring are a few reasons for the underutilisation of VKAs [6,7]. Studies have shown a correlation between the poor quality of anticoagulation and adverse events such as bleeding, thrombosis and death [8-11]. TTR is used as a measure to assess the adequacy of anticoagulation in patients taking VKA and has become a common reportable measure in anticoagulation outcome trials [12]. VKAs being highly effective with associated risk of bleeding qualify as an ideal target for quality improvement efforts.
Optimal outcomes can be ensured in patients receiving VKAs with a well-coordinated systematic evidence-based approach. This can be achieved by structured ACC. Majority of anticoagulated patients don’t receive care from ACCs in view of their non-availability. However, previous studies in India have been limited by sample size, focus on limited populations, variations in the clinical protocols and processes adopted with variable staffing model. Herein, the study report the experience of multifaceted ACC interventions on the quality of anticoagulation therapy in a tertiary care teaching hospital.
Materials and Methods
This retrospective study was conducted in Department of Cardiology, JSS hospital, Mysore, Karnataka, India. Three hundred and fifty two patients were enrolled in the ACC between February 2017 to April 2020.
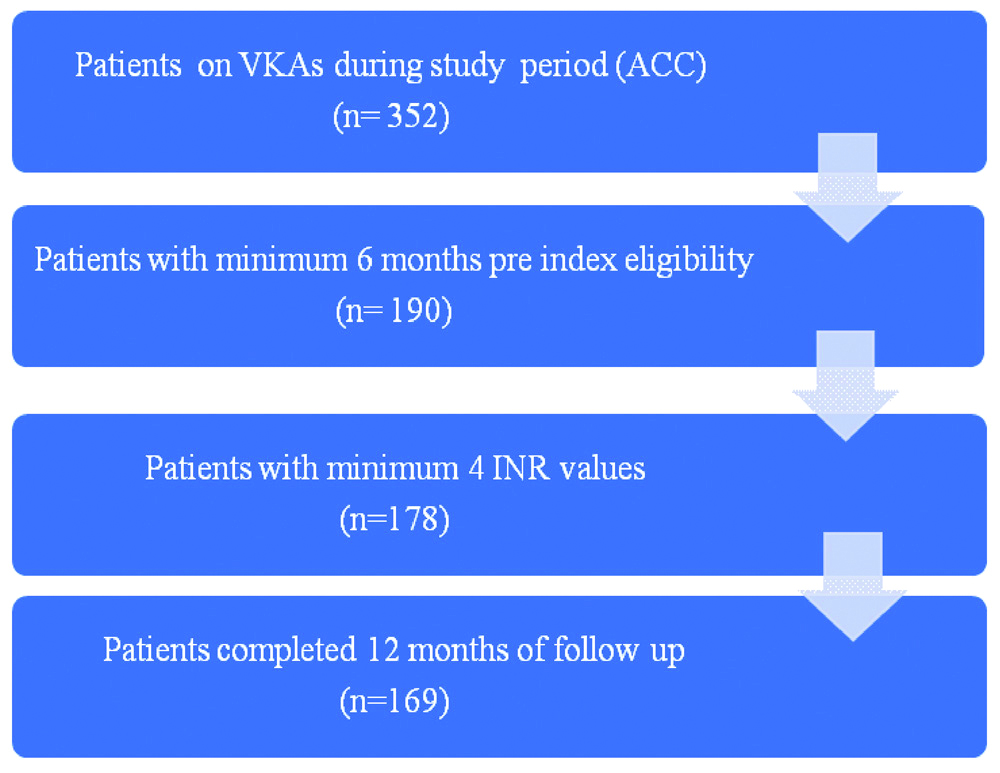
Inclusion criteria: The study were age >18 years, patients should have taken VKAs for a minimum period of six months with at least four INR values prior to enrolment in ACC and completed one year of follow-up. Of these 352 patients, 169 patients fulfilled the inclusion criteria and were analysed. Patients not fulfilling the inclusion criteria were excluded.
Exclusion criteria: The patient enrolment process is depicted in [Table/Fig-1]. Institutional Ethics Committee approval was taken to perform the study (JSSMC/IEC/2205/02/NCT/ER /2020-21). The informed consent was obtained from all the subjects at the time of enrolment in the ACC.
Participant enrolment process.
VKA: Vitamin K antagonist; ACC: Anticoagulation clinic; INR: International normalised ratio
Anticoagulation Clinic (ACC)
ACC was established in February 2017 in JSS Hospital, Mysore, Karnataka, India. It comprised of a Consultant cardiologist, clinical cardiologist, Clinical pharmacist (PharmD), Dietician and trained nursing staff. The Standard Operating Procedures (SOPs) were prepared based on the European Society of Cardiology (ESC) guidelines [13,14]. Any patient taking VKAs or requiring VKAs was eligible for referral to ACC. Patient data were entered in a specified format which included age, gender, socio-economic status, reason for anticoagulation, duration of anticoagulation, co-morbidities, other medications, CHA2DS2-VASc score, HASBLED score and SAMe-TT2R2 score [15-17]. Key aspects like patient education (VKA risks/benefits, possible diet/drug interactions), ordering relevant lab tests, titrating the dose of VKAs to achieve target INR, facilitating procedures which require interruption of VKAs, adverse events related to VKAs were addressed. Patients were telephonically reminded, in case if they missed a scheduled appointment.
Patient Centred Outcomes
The TTR, Time Over Range (TOR), Time Below Range (TBR) and percentage of PINRR were calculated by Rosendaal linear interpolation technique for each patient [18,19]. Therapeutic INR of 2-3 was considered for atrial fibrillation, venous thromboembolism, mechanical aortic valve and 2.5-3.5 for mechanical mitral valve. Calculations were performed with the assistance of a template made available by INR Pro for patients requiring therapeutic range of 2-3 [20]. Manual calculation of TTRs was done for patients requiring therapeutic range of 2.5-3.5. INR values obtained during temporary discontinuation of anticoagulation because of planned surgery or an interventional procedure were not considered for calculation of TTR.
Anticoagulation related quality measures were analysed at the time of enrolment and subsequently at 3, 6, 12 months of follow-up. Values obtained at the time of enrolment were considered as the baseline (pre-ACC care) and compared with subsequent values obtained during follow-up in ACC (ACC care). Major bleeding was defined by the International Society on Thrombosis and Haemostasis criteria [21]. Stroke/Systemic Embolic Events (SEE) was defined as the combined endpoints of ischaemic stroke, Transient Ischaemic Attack (TIA), and systemic embolic events. Adverse events were recorded at the time of enrolment as per the case records and subsequently during follow-up in ACC.
Statistical Analysis
Data were entered into MS OFFICE Excel 2019 and analysed using the IBM Statistical Package for the Social Sciences (SPSS) version 25 for Windows. Categorical data were presented as a count (n) and percentage (%). Continuous variables were presented as mean (Standard Deviation; SD) or median (Interquartile Range; IQR). The study used parametric Student’s t-test and non-parametric chi-square test for comparison of continuous variables between two independent samples. The study conducted regression analysis (linear and multivariate) to identify factors that were independently associated with suboptimal anticoagulation. The dependent variable was TTR (TTR <70.0% versus TTR ≥70.0%). Statistical differences were interpreted at 95% CI. The p-value <0.05 (two-sided) was considered as significant.
Results
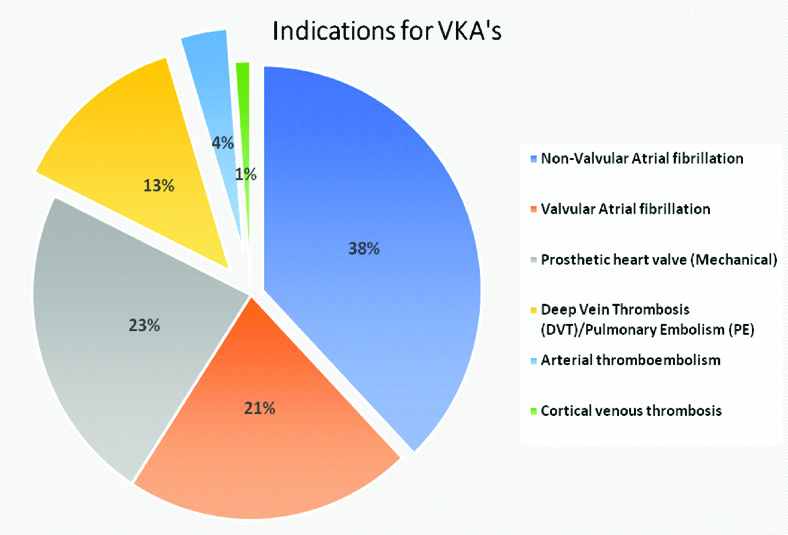
The mean age of the study population was 55.62±13.77 years. Hypertension was the most common comorbidity. Majority of the patients were illiterates (53.9%) and lived in rural areas (66.3%) [Table/Fig-2]. Acenocoumarol (n=158, 93.5%) was the most common VKA used. Atrial fibrillation (59%) was the most common indication for VKA [Table/Fig-3].
Patient characteristics (N=169).
| Parameter | Categories | Value |
|---|
| Age (years) | <60 | 103 (61%) |
| >61 | 66 (39%) |
| Gender | Male | 96 (56.8%) |
| Female | 73 (43.2%) |
| Literacy | Literate | 78 (46.1%) |
| Illiterate | 91 (53.9%) |
| Economic status | Upper class | 8 (4.7%) |
| Upper middle class | 36 (21.3%) |
| Lower middle class | 100 (59.2%) |
| Upper lower | 25 (14.8%) |
| Lower | 0 |
| Location | Urban | 57 (33.7%) |
| Rural | 112 (66.3%) |
| Smoking habit | Smokers | 16 (9.5%) |
| Nonsmokers | 153 (90.5%) |
| Alcohol use | 20 (11.8%) |
| Hypertension | 112 (66.3%) |
| Diabetes | 49 (29%) |
| Chronic heart failure | 41 (24.3%) |
| Vascular disease* | 39 (23%) |
| TIA/Stroke* | 12 (7.1%) |
| Chronic kidney disease | 19 (11.2%) |
| Medications** | Amiodarone | 44 (26%) |
| Antiplatelet | 43 (25.5%) |
| NSAIDs | 4 (2.4%) |
| HAS BLED score | Median (IQR) | 2 (1-3) |
| <3 | 98 (58%) |
| ≥3 | 71 (42%) |
*Ischaemic Heart Disease and Peripheral Arterial Disease, TIA: Transient ischaemic attack
**Medications: Drugs potential for drug interaction with VKAs
Indications for Vitamin-K Antagonists.
Patient Centred Outcomes
Mean TTRs were significantly higher in the ACC care when compared with the pre-ACC care (77.58±8.85% vs 51.01±16.7%, p<0.0001). As early as three months of follow-up in ACC, there was significant improvement in time spent in the therapeutic range (73.18±13.56 vs 51.01±16.7%, p<0.0001). Subsequent follow-up revealed a further increase in TTRs (75.54±8.32% at 6 months and 77.45±7.93% at 12-months, p=0.03).
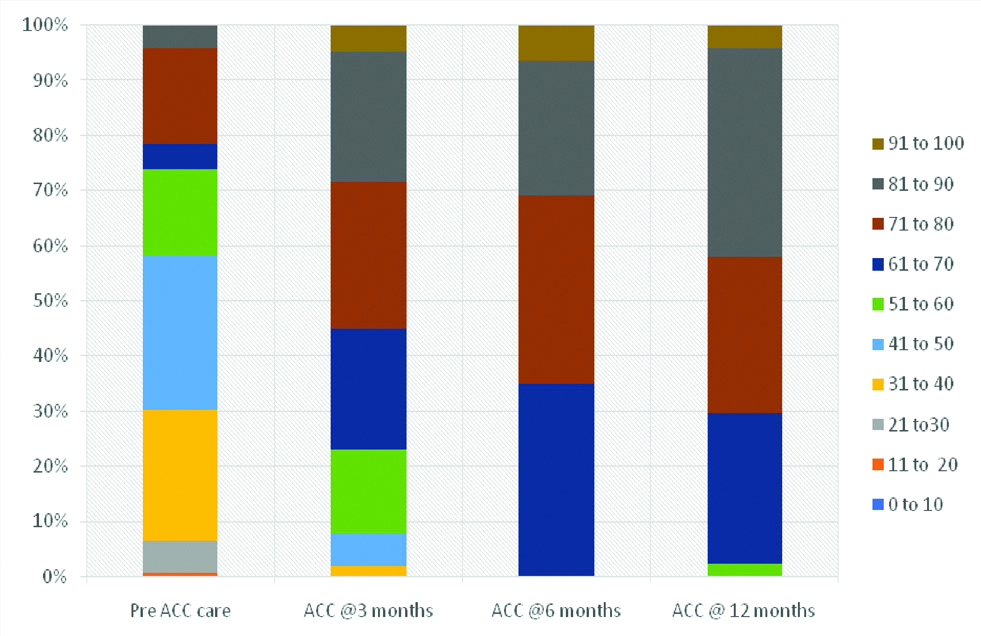
At the time of enrolment in ACC, 21.9% patients (n=37) with VKA experience had i-TTRs >70%. There was a significant improvement in i-TTRs at 6 months with ACC care which persisted on follow-up at 12 months [65.7% (n=111) and 70.4% (n=119) had i-TTRs >70% at 6 months and 12 months respectively]. The distribution of i-TTRs at baseline and at follow-up in ACC is depicted in [Table/Fig-4]. Patients in the ACC underwent INR testing more frequently than pre-ACC care in the first six months (11.4 INR tests/patient in ACC versus 5 INR tests/patient in pre ACC care). Percentage of tests over/ below the INR range was significantly lower in the ACC care than pre-ACC care. Time interval from sub therapeutic or supra therapeutic INR to the next INR testing was lower in the ACC care than the pre-ACC care [Table/Fig-5].
Distribution of (i-TTRs) at baseline and follow-up.
ACC: Anticoagulation clinic; i-TTR: Individual time in therapeutic range
Patient centred outcomes in Pre ACC care and ACC care (N=169).
| Parameter | Pre-ACC care (6 months) | ACC care (12 months) | p-value |
|---|
| TTR (%) | 51.01±16.70 | 77.58±8.85 | <0.001* |
| Interval (days) between INR tests (INR monitoring interval) | 26±18.5 | 15±11.2 | <0.001* |
| Interval (days) to next INR test after extreme INR(INR <1.5, INR >4.0) | 10±6.4 | 3±2.5 | <0.001* |
| PINRR | 41.7±15.24 | 69.68±11.50 | <0.001* |
| Test over range | 123 (14.5%) | 370 (9.8 %) | <0.001# |
| Test below range | 376 (44.4 %) | 582 (15.3%) | <0.001# |
| Number of INR draws | 845 | 3785 | - |
| Average number of INR draws/per patient in six months | 5 | 11.4 | - |
*Statistically significant p-value has been obtained by performing t-test. #Statistically significant p-value has been obtained by Chi-square test.
TTR: Time in therapeutic range; INR: International normalised ratio; PINRR: Percentage of international normalised ratio in the therapeutic range
In present study, 4.7% (n=8) of the patients had adverse events related to anticoagulant therapy with pre-ACC care and 2.4% (n=4) of patients had adverse events related to anticoagulant therapy with ACC care. Major bleeding and thromboembolic events were higher with pre-ACC care than ACC care (1.8% vs 0.6% and 2.36% vs 0.6%, respectively). Median INR was 7.2 in pre-ACC care and 5.5 in ACC care at the time of major bleeding. Median INR was 1.4 in pre-ACC care and 1.6 in ACC care at the time of thromboembolic event [Table/Fig-6].
| Pre-ACC care | ACC care | p-value |
|---|
| Major bleeding | | | |
| • GI bleeding | 2 (1.18%) | 1 (0.6%) | 0.563 |
| • IC bleed | 1 (0.59%) | 0 | 0.193 |
| Minor bleeding | 1 (0.59%) | 2 (1.18%) | 0.563 |
| Stroke/TIA | 3 (1.77%) | 1 (0.6%) | 0.315 |
| Recurrent DVT | 1 (0.59%) | 0 | 0.318 |
| Fatal events | 0 | 1 (0.59%) | 0.193 |
| Median INR at the time of major bleeding | 7.2 | 5.5 | - |
| Median INR at the time of thromboembolic event | 1.4 | 1.6 | - |
ACC: Anticoagulation clinic; GI bleeding: Gastro intestinal bleeding; IC bleed: Intra cranial bleeding; TIA: Transient ischemic attack; DVT: Deep vein thrombosis, INR: International normalised ratio
In univariate regression analysis, factors like illiteracy, HAS BLED score >3, concomitant medications like Amiodarone were associated with suboptimal anticoagulation [Table/Fig-7]. However, the same factors were found to be independently associated with suboptimal anticoagulation in multivariate logistic regression analysis [Table/Fig-8].
Linear regression analysis for predictors of poor anticoagulation control (TTR <70%) at 12 months follow-up.
| Parameter | df | F | p-value |
|---|
| Age (years) | 1 | 0.28 | 0.867 |
| Gender | 1 | 0.358 | 0.550 |
| Literacy | 1 | 18.688 | <0.001 |
| Economic status | 1 | 0.281 | 0.597 |
| Location | 1 | 0.563 | 0.454 |
| Hypertension | 1 | 0.005 | 0.945 |
| Diabetes | 1 | 0.788 | 0.376 |
| Chronic heart failure | 1 | 0.171 | 0.680 |
| Vascular disease | 1 | 0.004 | 0.951 |
| Medications |
| • Amiodarone | 1 | 7.321 | 0.008 |
| • Antiplatelets | 1 | 2.185 | 0.141 |
| HAS BLED score | 1 | 26.959 | <0.001 |
p value less than 0.05 statistically significant
Multivariate regression model for predictors of poor anticoagulation control (TTR <70%) at 12 months of follow-up.
| Parameter | Odds ratio | 95% CI | p-value |
|---|
| Literacy |
| Literate | Reference | 1.76-7.56 | 0.005 |
| Illiterate | 3.65 |
| MedicationAmiodarone | 12.26 | 4.73-31.80 | <0.001 |
| HAS BLED score |
| ≤3 | Reference | 1.98-7.61 | <0.001 |
| >3 | 3.89 |
p value less than 0.05 statistically significant
Discussion
In present study, patients receiving care in ACC had better control of anticoagulation in the form of greater time spent in therapeutic range (Mean TTR- 77.58%). However, mean TTRs achieved with pre-ACC care was 51.01%. Data has shown that Indian patients in ROCKET-AF study and Garfield-AF registry achieved mean TTR of 32.6% and 25.2%, respectively [22,23].
Multiple meta-analysis of randomised and real-world studies have demonstrated that TTRs and PINRRs were typically near or below 60% [24-26]. The European consensus document recommends TTR of >70% for optimal outcomes [27]. NICE guidelines recommend a TTR of >65% for patients with AF on VKA therapy [28]. Achieved TTRs in present study at the end of one year follow-up is above the proposed benchmark of >65-70%.
PINRR was low in the initial two months of follow-up in spite of all patients having prior VKA experience. The reason for this is multifactorial: majority of the patients are from rural area with high illiteracy rate who had difficulty in understanding benefits and side effects of VKAs, delay in INR testing or getting INR tested after stopping VKAs for 1-2 days quoting reason as running out of stock of medication, not buying medications in expectation of a possible dose change in next scheduled ACC visit. This issue was addressed and the patients were reinforced about the measures to be taken. Subsequently, mean TTRs showed a rising trend. There was a significant increase in the time spent in the therapeutic range at three months and the trend showed further improvement at six months and 12 months.
INR testing was done more frequently in pre-ACC care. INR test was performed once in a week till INR was in the therapeutic range and subsequently once in a month when 2 consecutive INRs were in the therapeutic range. The average time interval from out of range INR to next INR testing was lower in the ACC group when compared to pre-ACC care group. Because of this, patients in ACC group could spend more time in therapeutic range. Randomised controlled trials and studies related to ACCs documented better control of INR compared to community settings that were possible due to frequent monitoring, organised care and improved adherence to VKAs [29].
Multivariate logistic regression analysis revealed that the patient characteristics associated with suboptimal anticoagulation (TTR <70%) were illiteracy, HAS BLED score >3, and concomitant medication (Amiodarone). Studies have reported factors like female gender, young age, smoking, minority status, stroke history, Amiodarone use, heart failure, diabetes and others as predictors of low TTRs [30,31].
Several studies have validated TTR as a quantitative measure of the quality of anticoagulation control and as a predictor of bleeding and thromboembolic events [32-34].
Studies have demonstrated that patients managed in an ACC have better outcomes in terms of improved TTR, lower rates of major bleeding and thrombosis, and decreased health care costs than those managed in community practice [26,35-37]. The positive influence of multifaceted ACC care on TTRs and its impact on bleeding complications and thromboembolic events observed in index study corroborates with findings of previous research [26,35,36]. Present study has revealed that structured ACC comprising multidisciplinary team incorporating evidence-based guidelines can deliver superior patient centred outcomes.
Limitation(s)
In present study, patients with VKA experience enrolled in ACC were analysed. VKA naïve patients enrolled in ACC need to be analysed for assessing the overall impact on outcomes.
Conclusion(s)
Dedicated ACC services can facilitate achievement of optimal anticoagulation control as measured by TTR translating into better clinical outcomes.
*Ischaemic Heart Disease and Peripheral Arterial Disease, TIA: Transient ischaemic attack
**Medications: Drugs potential for drug interaction with VKAs
[1]. Reiffel JA, Time in the Therapeutic Range (TTR): An overly simplified conundrumJ Innov Cardiac Rhythm Manag 2017 8:2643-46.10.19102/icrm.2017.08030232494441 [Google Scholar] [CrossRef] [PubMed]
[2]. Hart RG, Halperin JL, Atrial fibrillation and thromboembolism: A decade of progress in stroke preventionAnnals of Internal Medicine 1999 131(9):688-95.10.7326/0003-4819-131-9-199911020-0001010577332 [Google Scholar] [CrossRef] [PubMed]
[3]. Gopalakrishnan C, Schoeneweis S, Bartels DB, Zint K, Santiago Ortiz A, Huybrechts KF, Evaluating utilisation patterns of oral anticoagulants in routine careJournal of Thrombosis and Haemostasis 2019 17(7):1033-43.10.1111/jth.1446731038824 [Google Scholar] [CrossRef] [PubMed]
[4]. Bussey H, Traditional anticoagulant therapy: Why abandon half a century of success?American Journal of Health-System Pharmacy 2002 59(suppl_6):S03-06.10.1093/ajhp/59.suppl_6.S312400243 [Google Scholar] [CrossRef] [PubMed]
[5]. Dlott JS, George RA, Huang X, Odeh M, Kaufman HW, Ansell J, Hylek EM, National assessment of warfarin anticoagulation therapy for stroke prevention in atrial fibrillationCirculation 2014 129(13):1407-14.10.1161/CIRCULATIONAHA.113.00260124493817 [Google Scholar] [CrossRef] [PubMed]
[6]. Bungard TJ, Ghali WA, Teo KK, McAlister FA, Tsuyuki RT, Why do patients with atrial fibrillation not receive warfarin?Archives of Internal Medicine 2000 160(1):41-46.10.1001/archinte.160.1.4110632303 [Google Scholar] [CrossRef] [PubMed]
[7]. Cohen N, Almoznino-Sarafian D, Alon I, Gorelik O, Koopfer M, Chachashvily S, Warfarin for stroke prevention still underused in atrial fibrillation: Patterns of omissionStroke 2000 31(6):1217-22.10.1161/01.STR.31.6.121710835435 [Google Scholar] [CrossRef] [PubMed]
[8]. Cancino RS, Hylek EM, Reisman JI, Rose AJ, Comparing patient-level and site-level anticoagulation control as predictors of adverse eventsThrombosis Research 2014 133(4):652-56.10.1016/j.thromres.2014.01.01324502961 [Google Scholar] [CrossRef] [PubMed]
[9]. Rose AJ, Berlowitz DR, Ash AS, Ozonoff A, Hylek EM, Goldhaber-Fiebert JD, The business case for quality improvement: oral anticoagulation for atrial fibrillationCirculation: Cardiovascular Quality and Outcomes 2011 4(4):416-24.10.1161/CIRCOUTCOMES.111.96059121712521 [Google Scholar] [CrossRef] [PubMed]
[10]. Ibrahim S, Jespersen J, Poller L, European Action on AnticoagulationThe clinical evaluation of international normalised ratio variability and control in conventional oral anticoagulant administration by use of the variance growth rateJournal of Thrombosis and Haemostasis 2013 11(8):1540-46.10.1111/jth.1232223945031 [Google Scholar] [CrossRef] [PubMed]
[11]. Lind M, Fahlén M, Kosiborod M, Eliasson B, Odén A, Variability of INR and its relationship with mortality, stroke, bleeding and hospitalisations in patients with atrial fibrillationThrombosis Research 2012 129(1):32-35.10.1016/j.thromres.2011.07.00421851969 [Google Scholar] [CrossRef] [PubMed]
[12]. Copplestone A, Roath S, Assessment of therapeutic control of anticoagulationActa Haematologica 1984 71(6):376-80.10.1159/0002066226433618 [Google Scholar] [CrossRef] [PubMed]
[13]. Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTSEuropean Journal of Cardio-Thoracic Surgery 2016 50(5):e1-88. [Google Scholar]
[14]. Authors/Task Force MembersKonstantinides SV, Torbicki A, Agnelli G, Danchin N, Fitzmaurice D, 2014 ESC Guidelines on the diagnosis and management of acute pulmonary embolism: The Task Force for the Diagnosis and Management of Acute Pulmonary Embolism of the European Society of Cardiology (ESC) Endorsed by the European Respiratory Society (ERS)European Heart Journal 2014 35(43):3033-80.10.1093/eurheartj/ehu28325173341 [Google Scholar] [CrossRef] [PubMed]
[15]. Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ, Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: The euro heart survey on atrial fibrillationChest 2010 Feb 137(2):263-72.Epub 2009 Sep 1710.1378/chest.09-158419762550 [Google Scholar] [CrossRef] [PubMed]
[16]. Pisters R, Lane DA, Nieuwlaat R, de Vos CB, Crijns HJ, Lip GY, A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: The Euro Heart SurveyChest 2010 Nov 138(5):1093-100.Epub 2010 Mar 1810.1378/chest.10-013420299623 [Google Scholar] [CrossRef] [PubMed]
[17]. Apostolakis S, Sullivan RM, Olshansky B, Lip GYH, Factors affecting quality of anticoagulation control among patients with atrial fibrillation on warfarin: The SAMe-TT2R2 scoreChest 2013 Nov 144(5):1555-1563.10.1378/chest.13-005423669885 [Google Scholar] [CrossRef] [PubMed]
[18]. Loeliger EA, Laboratory control, optimal therapeutic ranges and therapeutic quality control in oral anticoagulationActa Haematologica 1985 74(3):125-31.10.1159/0002061873938155 [Google Scholar] [CrossRef] [PubMed]
[19]. Rosendaal FR, Cannegieter SC, Van der Meer FJ, Briet E, A method to determine the optimal intensity of oral anticoagulant therapyThrombosis and Haemostasis 1993 70(03):236-39.10.1055/s-0038-16515878470047 [Google Scholar] [CrossRef] [PubMed]
[20]. INR Pro [website] Rosendaal method for % INR in range. INR Pro; Available from: www.inrpro.com/rosendaal.asp. [Accessed 2017 December 30] [Google Scholar]
[21]. Schulman S, Kearon C, Subcommittee on control of anticoagulation of the scientific and standardization committee of the international society on thrombosis and haemostasis. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patientsJ Thrombhaemost 2005 3(4):692-94.10.1111/j.1538-7836.2005.01204.x15842354 [Google Scholar] [CrossRef] [PubMed]
[22]. Singer DE, Hellkamp AS, Piccini JP, Mahaffey KW, Lokhnygina Y, Pan G, Impact of global geographic region on time in therapeutic range on warfarin anticoagulant therapy: Data from the ROCKET AF clinical trialJournal of the American Heart Association 2013 2(1):e00006710.1161/JAHA.112.000067 [Google Scholar] [CrossRef]
[23]. Sawhney JP, Kothiwale VA, Bisne V, Durgaprasad R, Jadhav P, Chopda M, Risk profiles and one-year outcomes of patients with newly diagnosed atrial fibrillation in India: Insights from the GARFIELD-AF RegistryIndian Heart Journal 2018 70(6):828-35.10.1016/j.ihj.2018.09.00130580852 [Google Scholar] [CrossRef] [PubMed]
[24]. Mearns ES, White CM, Kohn CG, Hawthorne J, Song JS, Meng J, Quality of vitamin K antagonist control and outcomes in atrial fibrillation patients: A meta-analysis and meta-regressionThrombosis Journal 2014 12(1):1410.1186/1477-9560-12-1425024644 [Google Scholar] [CrossRef] [PubMed]
[25]. Mearns ES, Kohn CG, Song JS, Hawthorne J, Meng J, White CM, Meta-analysis to assess the quality of international normalised ratio control and associated outcomes in venous thromboembolism patientsThrombosis Research 2014 134(2):310-19.10.1016/j.thromres.2014.05.03524935672 [Google Scholar] [CrossRef] [PubMed]
[26]. Erkens PM, Ten Cate H, Büller HR, Prins MH, Benchmark for time in therapeutic range in venous thromboembolism: A systematic review and meta-analysisPLoS One 2012 7(9)10.1371/journal.pone.004226923049730 [Google Scholar] [CrossRef] [PubMed]
[27]. Authors/Task Force MembersCamm AJ, Lip GY, De Caterina R, Savelieva I, Atar D, 2012 focused update of the ESC guidelines for the management of atrial fibrillation: an update of the 2010 ESC guidelines for the management of atrial fibrillation developed with the special contribution of the European Heart Rhythm AssociationEuropean Heart Journal 2012 33(21):2719-47.10.1093/eurheartj/ehs25322922413 [Google Scholar] [CrossRef] [PubMed]
[28]. National Clinical Guideline Centre (UK)Atrial Fibrillation: The Management of Atrial Fibrillation 2014 LondonNational Institute for Health and Care Excellence (UK) [Google Scholar]
[29]. Nelson WW, Damaraju CV, Lu L, Schein J, Fields LE, Wildgoose P, Conference Presentation. In Patterns of INR stability among newly initiated warfarin patients with NVAF 2013 Dallas TX [Google Scholar]
[30]. Laliberte F, Cloutier M, Nelson WW, Coleman CI, Pilon D, Olson WH, Real-world comparative effectiveness and safety of rivaroxaban and warfarin in nonvalvular atrial fibrillation patientsCurrent Medical Research and Opinion 2014 30(7):1317-25.10.1185/03007995.2014.90714024650301 [Google Scholar] [CrossRef] [PubMed]
[31]. Schein JR, White CM, Nelson WW, Kluger J, Mearns ES, Coleman CI, Vitamin K antagonist use: Evidence of the difficulty of achieving and maintaining target INR range and subsequent consequencesThrombosis Journal 2016 14(1):1410.1186/s12959-016-0088-y27303213 [Google Scholar] [CrossRef] [PubMed]
[32]. Nieuwlaat R, Connolly BJ, Hubers LM, Cuddy SM, Eikelboom JW, Yusuf S, Quality of individual INR control and the risk of stroke and bleeding events in atrial fibrillation patients: A nested case control analysis of the ACTIVE W studyThrombosis Research 2012 129(6):715-19.10.1016/j.thromres.2011.08.02421924760 [Google Scholar] [CrossRef] [PubMed]
[33]. Conolly SJ, Pogue J, Eikelboom J, Flaker G, Commerford P, Franzosi MG, Benefit of oral anticoagulant over antiplatelet therapy in atrial fibrillation depends on the quality of international normalised ratio control achieved by centres and countries as measured by time in therapeutic rangeCirculation 2008 118:2029-37.10.1161/CIRCULATIONAHA.107.75000018955670 [Google Scholar] [CrossRef] [PubMed]
[34]. Veeger NJ, Piersma-Wichers M, Tijssen JG, Hillege HL, van der Meer J, Individual time within target range in patients treated with vitamin K antagonists: main determinant of quality of anticoagulation and predictor of clinical outcome. A retrospective study of 2300 consecutive patients with venous thromboembolismBritish Journal of Haematology 2005 128(4):513-19.10.1111/j.1365-2141.2004.05348.x15686461 [Google Scholar] [CrossRef] [PubMed]
[35]. Chiquette E, Amato MG, Bussey HI, Comparison of an anticoagulation clinic with usual medical care: anticoagulation control, patient outcomes, and health care costsArchives of Internal Medicine 1998 158(15):1641-47.10.1001/archinte.158.15.16419701098 [Google Scholar] [CrossRef] [PubMed]
[36]. Witt DM, Sadler MA, Shanahan RL, Mazzoli G, Tillman DJ, Effect of a centralized clinical pharmacy anticoagulation service on the outcomes of anticoagulation therapyChest 2005 127(5):1515-22.10.1378/chest.127.5.151515888822 [Google Scholar] [CrossRef] [PubMed]
[37]. Garwood CL, Dumo P, Baringhaus SN, Laban KM, Quality of anticoagulation care in patients discharged from a pharmacist-managed anticoagulation clinic after stabilization of warfarin therapyPharmacotherapy: The Journal of Human Pharmacology and Drug Therapy 2008 28(1):20-26.10.1592/phco.28.1.2018154470 [Google Scholar] [CrossRef] [PubMed]