Efficacy and Safety of Rituximab in Immunobullous Diseases: A Retrospective Study from a Tertiary Care Centre of Nepal
Sudip Parajuli1, Jyoti Vidhan2, Dinesh Binod Pokhrel3, Upama Paudel4
1 Assistant Professor, Department of Dermatology and Venereology, Maharajgunj Medical Campus, Institute of Medicine, Nepal.
2 Resident, Department of Dermatology and Venereology, Maharajgunj Medical Campus, Institute of Medicine, Nepal.
3 Professor, Department of Dermatology and Venereology, Maharajgunj Medical Campus, Institute of Medicine, Nepal.
4 Associate Professor, Department of Dermatology and Venereology, Maharajgunj Medical Campus, Institute of Medicine, Nepal.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Upama Paudel, Associate Professor, Department of Dermatology and Venereology, Maharajgunj Medical Campus, Institute of Medicine, Nepal.
E-mail: upama_ups@yahoo.com
Introduction
Rituximab is effective and safe treatment of immunobullous disorders. There are variations in doses of drugs used in different studies and uncertainties on when to use it along with use of adjuvant therapies. Efficacy and safety of this drug has not been described in Nepalese population till date. Dermatologists have hesitation in starting this drug in immunobullous diseases because of lack of data on efficacy and safety.
Aim
To assess the efficacy and side effects of Rituximab therapy in treating immunobullous disorders in Nepalese patients.
Materials and Methods
This was a retrospective study of patients with immunobullous diseases treated with Rituximab in Dermatological ward of Tribhuvan University Teaching Hospital, Kathmandu, Nepal from May 2018 to August 2019. Data were analysed for duration of disease and treatment received before Rituximab therapy, duration of steroid used before Rituximab, adverse effects due to prolonged steroid use, time to remission from 1st Rituximab pulse, duration of remission, relapse, duration of steroid and adjuvant drug used post 1st pulse and adverse effects associated with Rituximab. SPSS version 20 was used for data entry and descriptive statistics was used for analysis of the data.
Results
Nine patients (Pemphigus Vulgaris-8 (PV-8), Bullous Pemphigoid-1 (BP-1) were treated with Rituximab. Seven were treated for refractory disease not controlled by conventional therapy and two received Rituximab as first-line therapy. The patients were under follow-up for 15-60 weeks (mean 31.89±15.62 weeks). Out of these nine patients, eight were free of lesions in one to eight weeks (mean 5.125±2 weeks) of first pulse. One patient with Oral Pemphigus had persistence of old lesions, however there were no new cutaneous lesions after first pulse. Adverse effects were seen in four patients that included infusion reaction in one and infection in three. There was relapse in one patient at last follow-up.
Conclusion
Rituximab is efficacious and is safe in treating immunobullous disorders in Nepalese Population.
Azathioprine, Bullous pemphigoid, Cyclophosphamide, Pemphigus, Steroids
Introduction
Immunobullous diseases are a rare group of intra-epidermal and sub-epidermal autoimmune blistering diseases and consist mostly of PV, Pemphigus Foliaceous (PF) and BP [1]. The incidence of these diseases vary in different countries and has been reported as 0.5 cases per million inhabitants in Germany, 4.4 cases per million population per year in India to 50 cases per million inhabitants in Saudi Arabia [2-4].
Corticosteroids remain the mainstay of these group of disorders and are combined with a steroid sparing agents like Azathioprine and Cyclophosphamide in order to reduce long term side effects of high dose of corticosteroids [1]. More commonly in clinical practice, metabolic complications like hyperglycaemia and hypertension have been seen with use of high dose of steroid in these patients in addition to other side effects like immunosuppression and risk of serious infections [1]. Furthermore, patients of PV, PF and BP despite being treated with corticosteroid and immunosuppressant, keep on developing new lesions without complete resolution of old lesions and demand for additional therapy.
Rituximab is a chimeric monoclonal antibody that targets CD20+ B cells causing their depletion and decreasing production of autoantibodies [5]. It has been recommended for the treatment of new cases of PV as well as patients not responding to conventional therapy [6]. A systematic review of sixteen-year history of Rituximab therapy concluded that it is effective in adult and childhood/juvenile PV with risk of not responding, exacerbation of disease, and development of fatal infections [7]. Recently, Rituximab has been included as a first-line treatment for moderate-to-severe Pemphigus [8]. In Nepal, there is reservation amongst Dermatologist to use this drug because of high cost and lack of experience along with unavailability on efficacy and safety data in Nepalese population, till date.
This study aimed to assess the efficacy, side-effect profile and safety of rituximab in Nepalese patients with immunobullous disorders. This is the first study which describes the use of Rituximab in PV, PF and BP patients in Nepal.
Materials and Methods
This was a retrospective review of records of patients with immunobullous diseases treated with Rituximab from May 2018 to August 2019 in Dermatological ward of Tribhuvan University Teaching Hospital, Kathmandu, Nepal. The study was approved by Institutional Review Committee of Institute of Medicine, Maharajgunj, Kathmandu (Reference letter no: 185/(6-11)076/077).
Data were analysed for duration of disease before receiving rituximab, treatment received before Rituximab, duration of steroid used, adverse effects associated with steroid use, time to remission (defined as complete healing of existing cutaneous and mucosal lesion with no new lesions) from first pulse, duration of remission, any relapse, duration of steroid and duration of adjuvant drugs used after first pulse and adverse effects due to Rituximab.
All the patients, after baseline investigations {Complete Blood Count (CBC), Urine routine examination (Urine r/e), Liver Function Tests (LFTs), Renal Function Tests (RFTs), Chest X-Ray, Hepatitis B surface antigen (HBs Ag), Hepatitis C antibody (HCV Ab) and after pre-medication with Loratadine, Paracetamol and Dexamethasone were subjected to injection Rituximab as per Rheumatoid Arthritis (RA) protocol (1 gm IV in 500 mL normal saline on day one and day 15 with additional 500 mg IV on 12th and 18th months) [9]. It was combined with Prednisolone (1 mg/kg/day) gradually tapered over 3-6 months duration. Azathioprine (50 mg twice daily) and other adjuvant drugs (Cyclophosphamide 50 mg twice daily) were continued for same duration as of steroids in those patients who were already on these drugs and were stopped by 3-6 months. Follow-up was done every 2 weekly for first month and then monthly and was focused on disease remission, relapse and development of any complications.
Statistical Analysis
Statistical Package for the Social Sciences (SPSS) version 20 was used for data entry and descriptive statistics was used for analysis of the data.
Results
There were nine cases of immunobullous disorders treated with Rituximab during this time period. Out of these nine patients (five men and four women, aged 19-69 years), eight had PV and one had BP. Diagnosis was made based upon clinical, histopathologic and direct immunofluorescence findings. Seven patients had refractory disease, not controlled by conventional therapy and remaining two were treated with Rituximab as first-line therapy. Amongst the patients of PV, six had received oral steroids with or without a steroid sparing agent for 5-24 months and two had received Dexamethasone-Azathioprine Pulses (DAP). Out of these seven patients, three had already developed Diabetes Mellitus (one patient after six DAP, 2nd one after 24 months of oral steroid, and 3rd one after 5 months of oral steroid) and one had developed hypertension after 12 months of steroids. The clinical characteristics of patients have been summarised in [Table/Fig-1].
Clinical characteristics of patients. (PV-Pemphigus vulgaris, BP-Bullous pemphigoid, and DM-Diabetes mellitus). Baseline investigations: (CBC-Complete blood count, HIV-Human immunodeficiency virus, HBsAg-Hepatitis B surface antigen, HCV Ab-Hepatitis C antibody, LFT-Liver function test, RFT-Renal function test, Urine r/e-Urine routine examination).
| Serial number | Age/Sex | Diagnosis | Baseline investigations (CBC, Chest X-Ray, HIV Elisa, HBsAg, HCV Ab, LFT, RFT, Blood pressure, Blood sugar, Urine r/e) | Duration of illness at time of giving Rituximab | Previous treatment | Duration of steroid use | Side effects of steroid |
|---|
| 1 | 65 y/F | PV | Within normal limit | 24 months | aPrednisolone, bCyclophosphamide, cAzathioprine | 12 months | None |
| 2 | 29 y/M | PV | Within normal limit | 48 months | dDexamethasone, Azathioprine pulse-14 cycles | 14 months | None |
| 3 | 32 y/M | PV | Normal except for raised blood sugar | 24 months | dDexamethasone Azathioprine pulse-8 cycles | 8 months | DM |
| 4 | 57 yrM | PV | Normal except for raised blood pressure | 24 months | aPrednisolone, bCyclophosphamide | 20 months | Hypertension |
| 5 | 34 y/M | PV | Normal except for raised blood sugar | 24 months | aPrednisolone | 24 months | DM |
| 6 | 30 y/F | PV | Within normal limit | 1 month | aPrednisolone, cAzathioprine | 1 month | None |
| 7 | 19 y/F | PV | Within normal limit | 16 months | aPrednisolone, cAzathioprine | 10 months | None |
| 8 | 64 y/M | PV | Normal except for raised blood sugar | 7 months | aPrednisolone | 6 months | DM |
| 9 | 69 y/F | BP | Within normal limit | 36 months | aPrednisolone, Deflazacort, Dapsone | 36 months, intermittent | None |
Dose of drugs used: aPrednisolone (1 mg/kg), bCyclophosphamide (50 mg bd), cAzathioprine (50 mg bd), dDexamethasone (100 mg IV in 5% Dextrose for 3 consecutive days every month) Azathioprine (50 mg bd) pulse
These nine patients were under variable duration of follow-up ranging from 15-60 weeks (mean 31.89±15.62 weeks) after first Rituximab pulse. In eight patients, the time to remission from first pulse was one to eight weeks (mean 5.125±2 weeks). One patient had healing oral lesion at 15 weeks follow-up after first pulse without any cutaneous lesions. One Patient with BP relapsed at 9 months whereas out of 8 cases of pemphigus, no relapse was seen in any patients at last follow-up (15 weeks to 60 weeks). The patient with BP who had relapsed was given 500 mg of Rituximab at 9 months and her next pulse schedule was changed to 6 months.
A patient of PV was given third dose of Rituximab at six months due to delay in giving second pulse because she had developed Herpes Zoster after first pulse. Overall, complete remission was seen in seven out of nine patients (77.77%) at last follow-up [Table/Fig-2a,b]. Infusion reaction (difficulty in breathing without chest sign) during first pulse was seen in one patient. This was managed with reduction of drip to previous rate at which there was no reaction and then slowly increasing the drip (by half of the defined rate) along with oxygen, single injection of hydrocortisone 100 mg iv, Single dose of Paracetamol 500 mg and loratadine 10 mg. The patient were closely monitored for vitals (Pulse, Blood Pressure, ECG, Oxygen saturation using cardiac monitor) after that, till the drip was over. One patient developed secondary bacterial infection of wounds (proven by pus culture) and one patient developed pneumonia by Klebseilla pneumoniae (proven by sputum culture and chest X-ray) after first pulse. Infections were managed with antibiotics and after resolution of infection; second pulse was given in these two patients after subsidence of signs and symptoms. One of the patient developed herpes zoster at 2nd week, which was managed by giving antivirals. The second pulse was delayed by 2 weeks in this patient. The details on remission, relapse, duration of follow-up and adverse effects has been summarised in [Table/Fig-3].
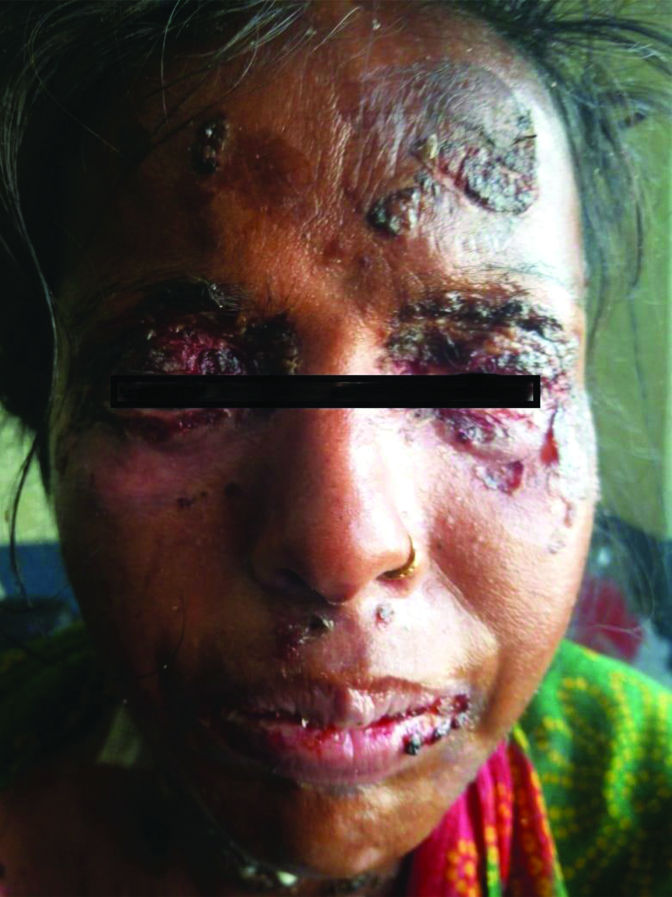
Crusted plaques of Pemphigus (pre-treatment).
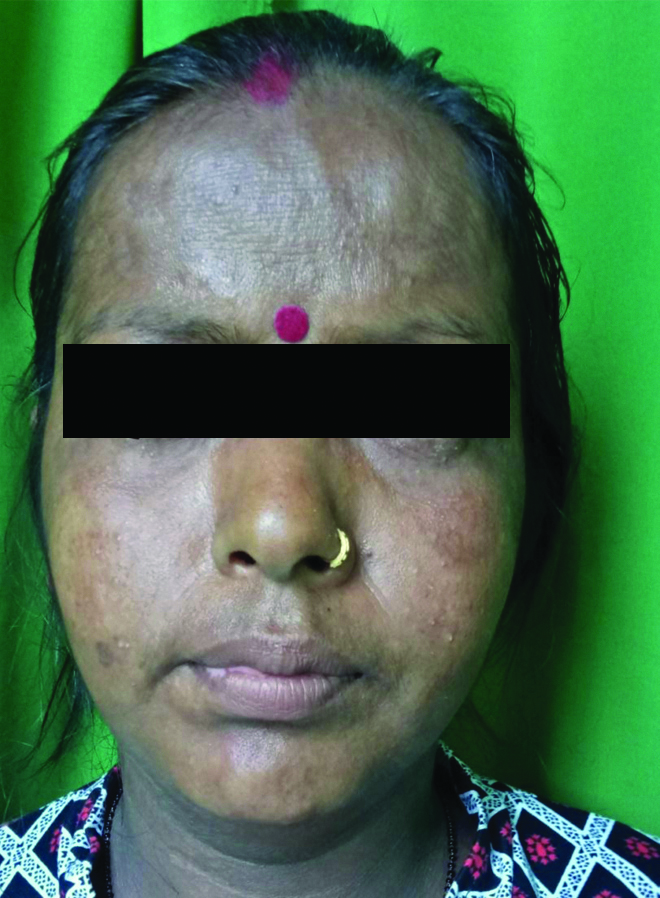
Healed lesions three months post-treatment.
Clinical details of patients on remission, relapse, and duration of follow-up and adverse effects.
| Serial number | Duration of follow-up from 1st pulse | Time to remission from 1st pulse | Duration of remission | Any relapse/time of relapse | Duration of steroid use post 1st pulse | Duration of adjuvant use post 1st pulse | Adverse effects related to Rituximab |
|---|
| 1 | 60 weeks | 7 weeks | 53 weeks | None | 16 weeks | 28 weeks | None |
| 2 | 55 weeks | 1 week | 54 weeks | None | 14 weeks | 14 weeks | Infusion reaction during 1st pulse |
| 3 | 34 weeks | 5 weeks | 29 weeks | None | 28 weeks | 24 weeks | Herpes zoster following 1st pulse |
| 4 | 26 weeks | 7 weeks | 19 weeks | None | 18 weeks | 14 weeks | None |
| 5 | 23 weeks | 5 weeks | 18 weeks | None | 16 weeks | 16 weeks | Secondary bacterial infection of wounds |
| 6 | 19 weeks | 4 weeks | 15 weeks | None | 10 weeks | 12 weeks | None |
| 7 | 16 weeks | 4 weeks | 12 weeks | None | 14 weeks | 9 weeks | None |
| 8 | 15 weeks | Oral lesions persisting | Not in remission till 15 weeks | Not applicable | 16 weeks | 2 weeks | Klebsiella pneumoniae pneumonia |
| 9 | 37 weeks | 8 weeks | 28 weeks | Relapsed at 36 weeks | 12 weeks | 12 weeks | Dyspnea at time of first pulse |
| Total | 285 weeks | Not applicable | Not applicable | Not applicable | 144 weeks | 131 weeks | Not applicable |
Discussion
Rituximab appears to be safe and efficacious in treatment of immunobullous diseases in Nepalese patients, though assessed in limited number of patients. In all of these patients, a good clinical response was seen both in terms of early disease control as well as decrease in total duration of steroid use. Adverse effects with short term follow-up were found in four patients and included infusion reactions, herpes reactivation and bacterial infections. One of the patients in this study with BP had relapsed after nine months while one patient had persistent oral lesions at 15 weeks after rituximab pulse. None of the patients died or had severe adverse effects at last follow-up (15 weeks to 60 weeks). Seven patients had received rituximab as second line therapy for uncontrolled disease or because of steroid induced side effects. These patients were tapered off steroids after Rituximab pulse without relapse of disease and subsequent disappearance of steroid induced side effects.
Rituximab was used as off label treatment for Pemphigus after reports of its efficacy in case series in pemphigus in 2007 [5,10,11]. In year 2017, Schmidt E described use of Rituximab as first-line treatment of Pemphigus with high level of evidence [12]. The major breakthrough study on rituximab use in pemphigus was done by Joly P et al., where the primary endpoint was the proportion of patients who achieved complete remission at 24 months [9]. It was seen that 89% assigned to Rituximab plus short term steroid therapy were in complete remission off-therapy versus 34% assigned to prednisolone alone with an absolute difference 55% points (95% CI 38.4-71.7; p<0.001) [9].
Till date, more than 600 patients have been treated by Rituximab for Pemphigus [9]. First series used the oncology regimen (375 mg/m2 weekly for 4 weeks per cycle), whereas latest series used the autoimmune regimen (two infusions of 1000 mg dose 2 weeks apart) [9].
Rituximab was used initially in recalcitrant Pemphigus with complete remission of 80%. There were few series which suggested a higher efficacy of Rituximab in patients treated early in the course of the disease. The rate of relapse in these series was about 20% after one year, and up to 50% at 6 years after Rituximab infusions, suggesting interest of maintenance infusions [13]. In this study, there was complete remission in 77.7% of cases at last follow-up (15 weeks to 60 weeks) similar to that reported by Joly P et al., of 80% [9]. There was only one case of relapse in case of BP (11.11%) at one year.
Limitation(s)
This study was carried out in limited number of patients and was retrospective in nature. The study suggested that Rituximab is relatively safe in the Nepalese population, however, still unsure due to short follow-up of the patients.
Conclusion(s)
Rituximab appears to be safe and efficacious in treating immunobullous disorders in Nepalese Population. The side-effects are less serious without any evidence of life-threatening adverse effects as observed in short course follow-up. At the same time, it reduces the total duration steroid use, thus decreasing the risk of steroid induced adverse effects.
Dose of drugs used: aPrednisolone (1 mg/kg), bCyclophosphamide (50 mg bd), cAzathioprine (50 mg bd), dDexamethasone (100 mg IV in 5% Dextrose for 3 consecutive days every month) Azathioprine (50 mg bd) pulse
Author Declaration:
Financial or Other Competing Interests: None
Was Ethics Committee Approval obtained for this study? Yes
Was informed consent obtained from the subjects involved in the study? NA
For any images presented appropriate consent has been obtained from the subjects. NA
Plagiarism Checking Methods: [Jain H et al.]
Plagiarism X-checker: Dec 21, 2019
Manual Googling: Jul 02, 2020
iThenticate Software: Aug 10, 2020 (9%)
[1]. Parajuli S, Paudel U, Dexamethasone Cyclophosphamide Pulse Therapy in Immunobullous Diseases: Our Experience Running Head: DC Pulse Therapy in Immnunobullous DiseasesJournal of BP Koirala Institute of Health Sciences [Internet] 2019 [cited 20 May 2020] 2(2):72-75.Available from: https://www.nepjol.info/index.php/jbpkihs/article/view/2787110.3126/jbpkihs.v2i2.27871 [Google Scholar] [CrossRef]
[2]. Bertram F, Broker EB, Zillikens D, Schimdt E, Prospective analysis of the incidence of autoimmune bullous disorder in lower Franconia, GermanyJ Dtsh Dermatol Ges 2009 7(5):434-39.10.1111/j.1610-0387.2008.06976.x19170813 [Google Scholar] [CrossRef] [PubMed]
[3]. Ajithkumar K, Incidence of pemphigus in Thrissur district, South IndiaIndian J Dermatol Venereol Leprol 2008 74(4):349-51.10.4103/0378-6323.4290118797055 [Google Scholar] [CrossRef] [PubMed]
[4]. Tallab T, Johari H, Bahamdan K, Karkashan E, Mourad M, Ibrahim K, The incidence of pemphigus in the southern region of Saudi ArabiaInt J Dermatol 2001 40(9):570-72.10.1046/j.1365-4362.2001.01247.x11737450 [Google Scholar] [CrossRef] [PubMed]
[5]. Ahmad AR, Spigelman Z, Cavacini LA, Posner MR, Treatment of Pemphigus Vulgaris with rituximab and intravenous immunoglobulinN Engl J Med 2006 355:1772-79.10.1056/NEJMoa06293017065638 [Google Scholar] [CrossRef] [PubMed]
[6]. Daneshpazhooh M, Balighi K, Mahmoudi H, Tavakolpour S, Abedini R, Soori T, Iranian guideline for rituximab therapy in pemphigus patientsDermatol Ther 2019 32(5):e1301610.1111/dth.13016 [Google Scholar] [CrossRef]
[7]. Tavakolpour S, Mahmoudi H, Balighi K, Abedini R, Daneshpazhooh M, Sixteen-year history of rituximab therapy for 1085 pemphigus vulgaris patients: A systematic reviewInt Immunopharmacol 2018 54:131-38.10.1016/j.intimp.2017.11.00529132070 [Google Scholar] [CrossRef] [PubMed]
[8]. Murrell DF, Peña S, Joly P, Marinovic B, Hashimoto T, Diaz LA, Diagnosis and management of pemphigus: Recommendations of an international panel of expertsJ Am Acad Dermatol 2020 82(3):575-85.e1.10.1016/j.jaad.2018.02.02129438767 [Google Scholar] [CrossRef] [PubMed]
[9]. Joly P, Maho-Vaillant M, Prost-Squaricioni C, Hebert V, Houivet E, Calbo S, First-line rituximab combined with short-term prednisolone versus prednisolone alone for the treatment of Pemphigus (Ritux-3): A prospective, multicenter, parallel-group, open-label randomized trialLancet 2017 389(10083):2031-40.10.1016/S0140-6736(17)30070-3 [Google Scholar] [CrossRef]
[10]. Joly P, Mouquet H, Rougeau JC, D’Incan M, Gilbert D, Jacquot S, A single cycle of rituximab for the treatment of severe pemphigusN Eng J Med 2007 357(6):545-52.10.1056/NEJMoa06775217687130 [Google Scholar] [CrossRef] [PubMed]
[11]. Schimdt E, Seitz CS, Benoit S, Brocker EB, Goebeler M, Rituximab in autoimmune bullous diseases: Mixed responses and adverse effectsBr J Dermatol 2007 156(2):352-56.10.1111/j.1365-2133.2006.07646.x17223877 [Google Scholar] [CrossRef] [PubMed]
[12]. Schmidt E, Rituximab as first-line for treatment of PemphigusLancet 2017 389(10083):1956-58.10.1016/S0140-6736(17)30787-0 [Google Scholar] [CrossRef]
[13]. Hebert V, Joly P, Rituximab in PemphigusImmunotherapy 2018 10(1):27-37.Epub 2017 Oct 2410.2217/imt-2017-010429064314 [Google Scholar] [CrossRef] [PubMed]