Laparoscopic Cholecystectomy (LC) is known to be associated with considerable postoperative pain [1]. Alleviation of postoperative pain can be attempted by using battery of pharmacological agents ranging from Nonsteroidal Anti-inflammatory Drugs (NSAIDs) to steroids to narcotics. Nevertheless, this attempt is often frustrating in case of postoperative nausea and vomiting. This has aptly been described as the “big little problem” by Kapur PA in an editorial review [2]. The occurrence of these morbidities postoperatively delays the discharge of the patients who are admitted for day care surgery. This not only adds to the woes of the patients, but also burdens the hospital resources, including bed occupancy, doctors’ time, nursing care and consumption of medications and disposables. In fact, the economic burden of postoperative morbidities and overstay is significant [3,4].
Thus, as the search for improving postoperative outcomes continues, this study was designed and conducted with a humble attempt to evaluate the efficacy of different volumes and dilutions of intraperitoneal lignocaine instillation, keeping the total dose same, as an adjunct to reduce postoperative morbidities. The primary outcome was postoperative VAS and secondary outcomes were vital parameters (heart rate, respiratory rate, mean arterial pressure, transcutaneous saturation), nausea and vomiting and length of hospital stay.
Materials and Methods
Study Design
Study was designed as a randomised controlled, double blinded study. The scheme has been shown in CONSORT 2010 flow chart in [Table/Fig-1]. The study was conducted in a teaching hospital in Sikkim, the north-east state of India. The duration of the study was 12 months spanning between May 2018 to April 2019. The study was approved by Institutional Research Protocol Evaluation Committee (IRPEC) with IRPEC number SMIMS/IRPEC/2018-78 dated 20th April 2018 and Institutional Ethics Committee (IEC) with IEC number SMIMS/IEC/2018-031 dated 26th May 2018.
CONSORT 2010 flow diagram for transparent reporting of trials.
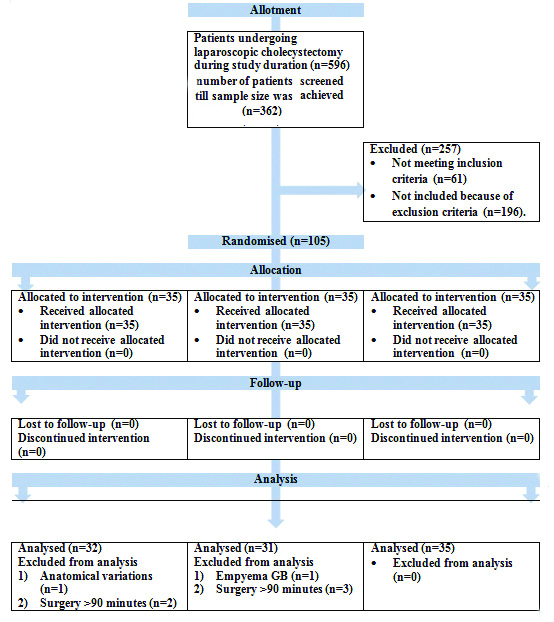
Total number of laparoscopic cholecystectomies done in the hospital during the study period was 596 with an average of 500-700 LC being done every year. Sample size was calculated for randomised comparison of three groups, for α-error of 5% (significance 0.05) and power of study (1-β) 90%. During the study period, 105 patients were enrolled for the study and were randomised into three groups (having 35 patients in each group).
Inclusion criteria: Patients undergoing elective LC for symptomatic cholelithiasis.
Exclusion criteria:
Complicated cholelithiasis
Empyema or mucocele of gall bladder
History of obstructive jaundice or patients who underwent Endoscopic Retrograde Cholangio Pancreatography (ERCP) or with Common Bile Duct (CBD) stent in situ
History of pancreatitis
Mirizzi’s syndrome
Anatomical variation in extra hepatic biliary anatomy
Significant bleeding from Gall Bladder (GB) fossa during surgery
Conversion to open cholecystectomy
Concomitant pathology in other organ found during surgery
Postoperative wound infection
Equipment or other technical failure leading to ergonomic difficulties
Any patient in whom drain was placed after surgery
Duration of surgery is >90 minutes
Any other co-morbidity other than diabetes and hypertension well controlled on medications.
Patient Randomisation and Blinding
Volumes and dilution to be given to patient were decided by a computer-generated random allocation list. The preparations of different volume and dilutions of lignocaine were made in operation room shortly before the start of the surgery depending on this allocation:
Group A: 5 mL 2% lignocaine+5 mL normal saline=100 mg lignocaine in 10 mL=10 mL of 1% lignocaine
Group B: 5 mL 2% lignocaine+100 mL of normal saline=100 mg lignocaine in 100 mL=100 mL of 0.1% lignocaine
Group C: 5 mL 2% lignocaine+500 mL normal saline=100 mg lignocaine in 500 mL=500 mL of 0.02% lignocaine
All patients were blinded with regard to the group they were assigned. Since blinding of the operating and anaesthesia team was not possible due to different volumes of the lignocaine used, they were not exposed to postoperative data till the end of the study. A separate team of observers recorded the postoperative data and they remained blinded regarding the group of the patient till the analysis of data completed by a separate team.
Consent for Randomisation and Blinding
All the patients and their relatives were explained about the process of randomisation and blinding. They were told if any patient in any of the group experienced more pain, they would be given rescue analgesia. They were also told that if they opt out of the study, they would receive standard care as per Department protocols. The median duration of session explaining all the procedure was 16 minutes.
Procedure Standardisation
Procedure was standardised and the scheme is shown in [Table/Fig-2].
Scheme followed during the study.
GB: Gall bladder; VAS: Visual analog score; HR: Heart rate; PONV: Postoperative nausea vomiting; MBP: Mean blood (arterial) pressure (MAP)
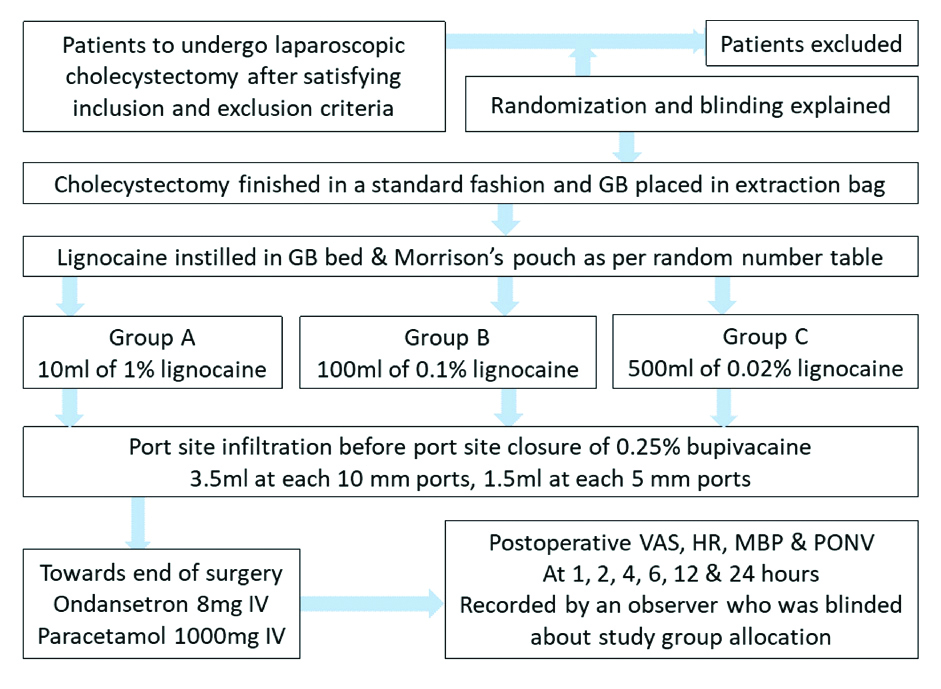
Operating surgeons neither collected the intra or postoperative data nor took part in its analysis. To keep the uniformity, all the cases were done by a single anaesthesiologist who was also never exposed to data collection or analysis. The instillation of lignocaine solution was done in Morrison’s pouch and GB bed area where dissection was carried out after bringing the patient in neutral/supine position. Extra solution was allowed to go spontaneously to other areas including right paracolic gutter, lesser sac and right sub-phrenic area. Rescue analgesia was also standardised. All the patients in study were given intravenous paracetamol (1 g) and ondansetron (4 mg) at 8th, 16th, and 24th hour. After that, all the injectables were stopped and the patients were given combination of aceclofenac (100 mg) and paracetamol (325 mg) twice a day. Intravenous aqueous diclofenac (75 mg) was offered as rescue analgesia followed by intravenous tramadol (50 mg) and ondansetron (4 mg) if not controlled by the former.
Statistical Analysis
Three groups were compared for postoperative pain (using VAS score), requirement of rescue analgesia, nausea and vomiting, vital parameters (heart rate, respiratory rate, mean arterial pressure, transcutaneous saturation) at 1, 2, 4, 6, 12 and 24 hours after surgery and hospital stay. Data was recorded in a proforma validated by experts from Department of Surgery and Anaesthesia. Analysis of Variance (ANOVA) was used to compare mean and χ2 test was used to compare categorical data. Tabulation and analysis were done in IBM©SPSS©21 using appropriate statistical tools.
cRESULTS
Statistical comparisons of demographic parameters were done to exclude any chance bias between population and sample. Female to male ratio in population sample was 3.22 and 2.66, respectively (p>0.05) and mean age was 41.17 and 39.87 years respectively (p>0.05).
A comparison of preoperative parameters has been presented in [Table/Fig-3] showing the similarities or differences among the three groups. The intraoperative findings have been mentioned in [Table/Fig-4].
Statistical comparisons of demographic parameters and morbidity among the three groups.
| Group A (n=32) | Group B (n=31) | Group C (n=35) | p-value |
|---|
| Age (Years) | 39.75 | 40.23 | 39.66 | 0.975 (ANOVA) |
| Gender (M:F) | 1:1.9 | 1:3.4 | 1:2.8 | 0.552 (χ2-test) |
| Body mass index (kg/m2) | 22.85 | 20.95 | 23.47 | 0.113 (ANOVA) |
| Hypertension | 1 (3.1%) | 3 (9.6%) | 1 (2.8%) | >0.375 (χ2-test) |
| Diabetes mellitus | 1 (3.1%) | 1 (3.2%) | 2 (5.7%) | >0.831 (χ2-test) |
| Observation | Group A | Group B | Group C | Total | Significance |
|---|
| Adhesions | Liver to AAW | 1 (3.12%) | 2 (6.45%) | 1 (2.85%) | 4 (4.08%) | 0.721 (χ2-test)* |
| GB to omentum | 5 (15.6%) | 5 (16.1%) | 5 (14.2%) | 15 (15.3%) |
| GB to bowel | 2 (6.25%) | 2 (6.45%) | 3 (8.57%) | 7 (7.14%) |
| GB to AAW | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Adhesion (Overall) | 8 (8.16%) | 9 (9.18%) | 9 (9.18%) | 26 (26.5%) |
| Bile spillage | 8 (25.0%) | 9 (29.0%) | 12 (34.2%) | 29 (29.8%) | 0.705 (χ2-test) |
| Stone spillage | 2 (2.0%) | 6 (6.12%) | 3 (3.06%) | 11 (11.2%) | 0.212 (χ2-test) |
| Mean duration of surgery (min) mean±SD | 50.78± 20.36 | 45.16± 15.24 | 52.43± 20.62 | 49.59± 19.05 | 0.279 (ANOVA) |
AAW: Anterior abdominal wall; GB: Gall bladder
*The 3 groups are compared based on overall presence of adhesions.
Postoperative Pain
The mean VAS of group B (100 mg lignocaine in 100 mL of normal saline) was lower than the overall mean VAS at different postoperative time intervals and consistently lower than those of groups A and C. The difference was statistically significant {p-value (ANOVA) <0.05} at all postoperative intervals it was assessed. This was followed by group A (100 mg lignocaine in 10 mL of normal saline) while group C (100 mg lignocaine in 500 mL of normal saline) fared the worst. A post-hoc analysis of pain between genders and different age groups did not show any significant differences. The study found a consistent (r=0.15 to 0.33) and significant (p<0.05 at all-time intervals) positive correlation between pain and duration of surgery. A subset analysis for each of the three groups for all time intervals though showed positive correlation but the values were not significant. An important observation was that in group B, correlation was negative. As the value of VAS was significantly lower in immediate postoperative period, it may imply that the patient with longer duration of surgery had the maximum beneficiary of dilution and volume used in group B (100 mg lignocaine in 100 mL normal saline) [Table/Fig-5].
Postoperative parameters.
| Group A | Group B | Group C | Overall | p-value |
|---|
| Pain (Visual analog scale: score out of 10) |
| 1 h | 5.00±1.368 | 4.77±1.087 | 5.94±1.514 | 5.27±1.425 | 0.001 |
| 2 h | 4.47±1.626 | 3.94±1.181 | 5.11±1.471 | 4.53±1.507 | 0.005 |
| 4 h | 4.44±1.900 | 3.68±1.249 | 4.71±1.487 | 4.30±1.613 | 0.026 |
| 6 h | 3.84±1.687 | 3.16±1.098 | 4.29±1.601 | 3.79±1.548 | 0.011 |
| 12 h | 3.41±1.624 | 2.58±0.807 | 3.74±1.771 | 3.27±1.544 | 0.007 |
| 24 h | 2.81±1.176 | 2.48±1.208 | 3.40±1.701 | 2.9±1.434 | 0.029 |
| Pulse rate (per minute) |
| 1 h | 82.19±11.169 | 80.00±9.94 | 80.46±9.663 | 80.88±10.199 | 0.669 |
| 2 h | 82.69±11.044 | 79.55±6.999 | 80.29±10.072 | 80.84±9.563 | 0.395 |
| 4 h | 82.59±9.238 | 77.58±4.603 | 81.23±8.735 | 80.52±8.058 | 0.037 |
| 6 h | 82.13±10.773 | 77.00±6.414 | 79.51±10.717 | 79.57±9.712 | 0.111 |
| 12 h | 79.66±9.22 | 77.74±6.382 | 80.31±8.235 | 79.29±8.045 | 0.415 |
| 24 h | 80.19±8.593 | 79.42±8.33 | 81.23±8.732 | 80.32±8.505 | 0.690 |
| Mean arterial pressure (mmHg) |
| 1 h | 88.71±10.816 | 87.37±11.917 | 87.29±11.109 | 87.78±11.180 | 0.850 |
| 2 h | 88.41±6.971 | 86.84±7.416 | 86.66±6.571 | 87.29±6.949 | 0.541 |
| 4 h | 88.58±6.999 | 88.52±7.011 | 87.51±6.677 | 88.18±6.837 | 0.772 |
| 6 h | 88.08±6.461 | 87.61±6.572 | 87.6±6.370 | 87.76±6.401 | 0.943 |
| 12 h | 89.58±6.707 | 89.07±8.58 | 87.5±7.743 | 88.67±7.680 | 0.513 |
| 24 h | 89.31±7.759 | 89.44±6.803 | 88.36±7.323 | 89.01±7.252 | 0.804 |
| Nausea and vomiting (number of patients) |
| 1 h | 1 (3.1%)* | - | - | 1 (1.0%)1 | 0.353 |
| 2 h | - | - | - | - | - |
| 4 h | - | 3 (9.6%)# | - | 3 (3.1%)1 | 0.353 |
| 6 h | - | 2 (6.5%)# | - | 2 (2.0%)1 | 0.110 |
| 12 h | - | - | - | - | - |
| 24 h | - | - | - | - | - |
| Respiratory rate (per minute) |
| 1 h | 16.69 | 20.23 | 19.46 | 18.80 | 0.385 |
| 2 h | 17.47 | 18.26 | 17.89 | 17.87 | 0.450 |
| 4 h | 17.38 | 18.90 | 18.23 | 18.16 | 0.077 |
| 6 h | 17.84 | 18.84 | 18.23 | 18.16 | 0.262 |
| 12 h | 17.91 | 19.45 | 18.57 | 18.63 | 0.600 |
| 24 h | 17.84 | 18.87 | 18.26 | 18.32 | 0.314 |
| Transcutaneous oxygen saturation (SpO2) |
| 1 h | 91.97 | 94.87 | 93.63 | 93.48 | 0.779 |
| 2 h | 96.06 | 96.29 | 96.06 | 96.13 | 0.854 |
| 4 h | 95.28 | 95.77 | 95.46 | 95.5 | 0.662 |
| 6 h | 95.22 | 95.19 | 95.89 | 95.45 | 0.489 |
| 12 h | 95.66 | 95.58 | 96.03 | 95.77 | 0.737 |
| 24 h | 95.44 | 95.19 | 96.23 | 95.64 | 0.099 |
The first two readings (1st and 2nd hour) were recorded in post anaesthesia care unit (PACU). Remaining 4 readings were taken in surgical wards. 1The numbers are exclusive to each row and are not to be summed up for that column. Total of 4 patients actually had nausea, *1 in group A and #3 in group B. Two out of three patients who felt nausea at 4th hour continued to have it at 6th hour too.
Vital Parameters
Pulse rate, as a surrogate for postoperative pain, was least in all postoperative time and significantly lower at 4th hour. The difference in mean arterial pressures and respiratory rate among the three groups was not significant statistically [Table/Fig-5].
Nausea and Vomiting
There were only 4 instances of nausea and none of vomiting in all three groups together and the finding was insignificant [Table/Fig-5].
Rescue Analgesia
The difference in the requirement of rescue analgesia was significant as shown in [Table/Fig-6].
Rescue analgesia (RA) 13 required RA on same day, 5 on next day. 2Both required RA next day. 31 required RA same day, 1 next day.
| Group A | Group B | Group C | Overall | p-value |
|---|
| Given | 8 (25%)1 | 2 (6.45%)2 | 2 (5.71%)3 | 12 (12.24%) | 0.027 (χ2 test) |
| Not given | 24 (75%) | 29 (93.55%) | 33 (94.29%) | 86 (93.88%) |
Day of Discharge
The data presented in [Table/Fig-7] seems to be conflicting as the patients who experienced least pain stayed in the hospital for a longer time. But the data needs to be interpreted along with socio-geographic data. The area is a hilly terrain and there are only two hospitals in the state of four districts offering surgeries. So, the hospital stay or discharge is invariably affected by the fact how far the patient belong to and how much patient requires to walk and climb from the road to reach the home.
| Group A | Group B | Group C | Overall | p-value |
|---|
| POD 1 | 4 (11.42%) | 5 (16.12%) | 5 (14.28%) | 14 (14.28%) | 0.669(χ2 test) |
| POD 2 | 27 (84.37%) | 22 (70.96%) | 27 (77.14%) | 76 (77.55%) |
| POD 3 | 1 (3.12%) | 4 (12.90%) | 3 (8.57%) | 8 (8.16%) |
POD: Postoperative day
Discussion
As various studies around the globe concentrate more on diversity of agents as well as timing, this study demonstrates the efficacy to differ for different volumes and dilutions for a similar dose of drug (lignocaine in present study which was 5 mL of 2% solution amounting to 100 mg of lignocaine). This study showed that a 100 mL solution of 5 mL of 2% lignocaine is significantly better than a 10 mL solution or a 500 mL solution of a similar dose of lignocaine in alleviating pain up to 24 hours postoperatively after LC. The only study which comes close to this one is that by Al-Kizwini GAM which compared two different strengths of lignocaine and found similar results against placebo but could not find any difference between the two intervention groups [5]. Agrawal S and Pai S did not find any difference between placebo and intraperitoneal analgesia [6]. Murdoch J et al., compared intravenous or intraperitoneal analgesia in controlling postoperating pain and told that both were equally effective analgesic when compared to placebo but did not differ significantly when compared against each other [7]. Roberts KJ et al., inferred that the benefits of elective LC in terms of controlling postoperative pain could not be extended to emergency LC [8]. Most of the studies found that intraperitoneal instillation of local anaesthetic agents did help in experiencing less postoperative pain as well as decreases the requirement of postoperative analgesia [9-14]. A summary of the review of all these studies has been presented in [Table/Fig-8] [5-14].
Summary of the articles discussed in the paper [5-14].
| Study | Study design | Salient features |
|---|
| This study(2018-19) | RCT, DB, 3 groups, n=105G1: 5 mL 2% Lignocaine + 5 mL NSG2: 5 mL 2% Lignocaine + 100 mL NSG3: 5 mL 2% Lignocaine + 500 mL NSLWI in all 3 groups with 0.25% Bupivacaine, 3.5 mL at each 10 mm, 1.5 mL at each 5 mm port site | Lignocaine dose was same (5 mL of 2% Lignocaine=100 mg)VAS as well as demand for rescue analgesia was significantly less at all postoperative durations in group 2 compared to group 1 and group 3. |
| Al-Kizwini GAM (2017) [5] | RCT, NB, 3 groups, n=110G1: Normal salineG2: 3 mL of 2% Lignocaine + 7 mL NS (0.6%)G3: 5 mL of 2% Lignocaine + 5 mL NS (1%) | Group 2 was significantly different in number of patients for severe pain (VAS) (p=0.018) and rescue analgesia (p=0.032) from group 1 but not from group 3 (p=0.142 and 0.063, respectively). |
| Agrawal S and Pai S (2017) [6] | RCT, 2 groups, n=50G1: Bupivacaine1 10 mL IP, 10 mL SD (total 100 mg)G2: Saline 10 mL IP, 10 mL SD | The difference in VAS, VRS and requirement of rescue analgesia at all postoperative hours in both the groups was not significant. |
| Murdoch J et al., (2016) [7] | RCT, DB, 3 groups, n=120G1: IP Ketorolac (30 mg/250 mL) + IV saline (1 mL)G2: IP NS (250 mL) + IV Ketorolac (30 mg/1 mL)G3: IP NS (250 mL) + IV NS (1 mL) | Group 1 and group 2 required significantly less postoperative fentanyl with an increase in mean time to first request for analgesia compared to group 3.There was no difference between group1 and group 2 for same parameters implying no difference between IP or IV ketorolac in LC. |
| Roberts KJ et al., (2013) [8] | RCT, DB, 2 groups, n=41Patients selected had acute cholecystitis and underwent emergency surgery.G1: BupivacaineG2: Saline | Emergency LC needs longer operative duration, more frequent washings and most of the patients are still under ongoing inflammation.No significant difference was found in postoperative pain scores (VAS), theatre recovery, analgesia requirement, respiratory rate, oxygen saturation, duration to ambulation, eating, satisfaction score and time to discharge.They concluded that the benefit of IP analgesia obtained in elective LC cannot be extended to emergency LC. |
| Memedov C et al., (2010) [9] | RCT, 3 groups, n=45G1: 150 mg/80 mL RopivacaineG2: 16 mg/80 mL LornoxicamG3: 80 mL NS | Patient-Controlled Analgesia (PCA) pump was used to deliver tramadol in the postoperative period and assessed pain while at rest, on coughing and on mobilisation.At the end of 24 hours, VAS was significantly lower in group 1 and group 2. Requirement of tramadol in postoperative duration was also significantly less in both the intervention groups compared to placebo.There was no difference between group 1 and 2 for the same parameters. |
| Golubovic S et al., (2009) [10] | RCT, 3 groups, n=90G1: SalineG2: 0.25% BupivacaineG3: 0.25% Bupivacaine + 100 mg Tramadol | Postoperative pain scores and rescue analgesia requirement were significantly lower in group receiving the IP bupivacaine alone or in combination with tramadol compared to saline group.There was no difference for the similar parameters between group 2 and group 3. |
| Lepner U et al., (2003) [11] | RCT, 4 groups, n=80G1: No LWI + No IPG2: 80 mL 0.125% Bupivacaine2 LWIG3: 80 mL NS LWIG4: 80 mL 0.125% Bupivacaine2 LWI + 0.15% Lignocaine 200 mL IP | Though different but on most of the occasions, the postoperative abdominal pain was significantly less in group 2 compared to all other groups.There was a paradox that the pain in group 2 was less compared to group 4 even though the patients in both the groups received bupivacaine and phenylephrine locally and the group 4 also received intraperitoneal lignocaine. |
| Maestroni U et al., (2002) [12] | RCT, 2 groups, n=60G1: 200 mL NSG2: 5 mg/kg Ropivacaine 200 mL | Pre-emptive intraperitoneal instillationVAS, VRS and stress response data was significantly lower in group 2 compared to group 1 |
| Bhardwaj N et al., (2002) [13] | RCT, 2 groups, DB, n=40G1: 20 mL NSG2: 0.5% Bupivacaine1 20 mL | Pain perception on both the pain scales and analgesia requirement was significantly less in group 2, on most of the occasions.No patient complained of shoulder pain in group 2. |
| Pasqualucci A et al., (1996) [14] | RCT, DB, n=120G1: 20 mL NS (before, after)G2: 20 mL NS (before) + 20 mL Bupivacaine1 (after)G3: 20 mL Bupivacaine1 (before/ after)G4: 20 mL Bupivacaine1 (before) + 20 mL NS (after) | VAS was significantly less in group 2, group 3 and group 4 compared to group 1. VAS was also less in group 3 and group 4 compared to group 2.Plasma glucose and cortisol concentration before and after surgery were significantly lower in group C.Data related to heart rate, respiratory rate and blood pressure did not correlate with each other, however they showed a positive correlation with pain perception. |
RCT: Randomised controlled trial; VRS: Verbal rating scale; DB: Double blinded; NB: Non-blinded; LWI: Local wound infiltration; IP: Intraperitoneal instillation; SD: Subdiaphragmatic 10.5% bupivacaine and 1:200,000 epinephrine 20.125% bupivacaine and 5 mg phenylephrine. LC: Laparoscopic cholecystectomy; NS: Normal saline. All intraperitoneal instillations are after cholecystectomy, before removal of port cannulas unless mentioned otherwise.
Limitation(s)
The study lacks the immediate post discharge as well as long term follow-up data of the patients. Pain was assessed only by VAS and no other scale or method was applied. The study did not assess the overall effect of pain relief on health related quality of life as well as return of normal activity.
Conclusion(s)
The study concluded that the different concentration (or dilution) and volume of a similar dose of intraperitoneal lignocaine instillation have different analgesic effect in postoperative period following LC in patients of symptomatic uncomplicated cholelithiasis. For a 100 mg total dose of lignocaine, 100 mL solution is more effective compared to 10 mL or 500 mL solution.
AAW: Anterior abdominal wall; GB: Gall bladder
*The 3 groups are compared based on overall presence of adhesions.
RCT: Randomised controlled trial; VRS: Verbal rating scale; DB: Double blinded; NB: Non-blinded; LWI: Local wound infiltration; IP: Intraperitoneal instillation; SD: Subdiaphragmatic 10.5% bupivacaine and 1:200,000 epinephrine 20.125% bupivacaine and 5 mg phenylephrine. LC: Laparoscopic cholecystectomy; NS: Normal saline. All intraperitoneal instillations are after cholecystectomy, before removal of port cannulas unless mentioned otherwise.