Audiological Findings in Diffused Axonal Injury Secondary to Road Traffic Accident
Mohammed Eliyas1, Abishek Umashankar2, G Amritha3
1 Clinical Audiologist, Department of Audiology, MERF Institute of Speech and Hearing (p) Ltd., Chennai, Tamil Nadu, India.
2 Postgraduate, Department of Audiology, MERF Institute of Speech and Hearing (p) Ltd., Chennai, Tamil Nadu, India.
3 Audiologist, Department of Audiology, MERF Institute of Speech and Hearing (p) Ltd., Chennai, Tamil Nadu, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Mrs. G Amritha, Old No. 1/1, Second Loop Street, South Canal Bank Road, Mandaveli, Chennai-28, Tamil Nadu, India.
E-mail: amritha13g@gmail.com
Diffused Axonal Injury (DAI) is a form of mild Traumatic Brain Injury (TBI) that occurs when there are rapid acceleration and deceleration of the head caused by road traffic accidents. It results in the accumulation of Amyloid Precursor Protein (APP) and increased calcium that causes damage to the axonal cytoskeleton and ion channels, hence resulting in degeneration. This report is of 35 year old male patient with DAI secondary to road traffic accident, where a complete audiological test battery was done. Upon multiple Computed Tomography (CT) investigations, the third CT findings revealed a hypodensity at the level of upper brainstem. Upon audiological evaluation, the patient had a moderate sensorineural hearing loss in the left ear and a mild sensorineural hearing loss in the right ear. The speech discrimination scores were poor in both the ears, thus suggesting a presence of a Retrocochlear involvement. On administering Auditory Brainstem Response (ABR), no V peak could be visualised in both ears, thus indicating a lesion at the upper brainstem which correlated with CT findings and on administering various other test batteries, findings revealed a presence of retrocochlear involvement. This study highlights the importance of carrying out both Electrophysiological and Radiological test procedures in diagnosing DAI.
Amyloid precursor protein, Auditory evoked potential, Brainstem, Radiology, Traumatic brain injury
Case Report
A 35-year-old male presented to the Department of Audiology and Speech Language Pathology on 10/01/2019, with the complaint of difficulty to understand speech and slurred speech since six months. The medical history revealed that the complaints were consequent to a road traffic accident, which took place on 15/07/2018. The reports reveal that the client had a loss of consciousness, nasal bleed, seizure and he was HBsAg positive. He was admitted to emergency care immediately and was under a coma for seven days. The patient had scalp avulsion for which he was under general anaesthesia and suturing was done for scalp avulsion. He was under mechanical ventilation and had undergone tracheostomy as an emergency procedure. The patient was later provided with various treatments that included drugs of Antiepileptic, Antibiotic, Analgesic, Antimetic, Antacids, with Antioedema measures which also included multivitamins and supportive treatments. Tracheostomy was closed after 13 days. The patient had undergone multiple CT procedures, the third CT revealed well defined hypo-density in the dorsal aspect of the midbrain. During follow-up, another CT was done that revealed resolving hypodensity in the brainstem, with no evidence of intra/extra haemorrhage. Thus, he was diagnosed with DAI and diffuse cerebral oedema.
At the time of presentation, a subjective and objective audiological test battery was performed on the patient. Pure tone audiometry was done using Cello inventis, Immitance audiometry was done using Clarinet inventis. Physiological tests were done using intelligent hearing system smart Evoked Potentials (EP), hearing aid trial was done in piano inventis using high gain hearing aids and Frequency Modulation (FM) systems. Tuning fork test revealed rinne positive for both ears and weber lateralising towards right. On undergoing pure tone audiometry and Speech audiometry it was found that right ear had mild sensorineural hearing loss and left ear had moderate sensorineural hearing loss [Table/Fig-1]. Speech audiometry revealed 0% speech discrimination scores. He had bilateral As type tympanograms on Impedance audiometry testing, but there were retrocochlear pathological findings on the tone decay test. Oto Acoustic Emission test revealed absent Distortion Product Oto Acoustic Emission in both ears [Table/Fig-2]. ABR results showed no V peaks as there was no replicability, even though there was a peak; like in right ear the wave morphology was poor to be called a peak; I peak and III peaks being replicable in both ears [Table/Fig-3]. Late Latency Response (LLR) revealed P1, P2, N1 and N2 peaks being present in both ears at normal absolute latencies [Table/Fig-4]. No peaks could be seen in Mismatch Negativity and P300. The patient was fitted with hearing aids and FM system to check the benefit during hearing aid trial and FM trial and did not benefit from hearing aids and FM system.
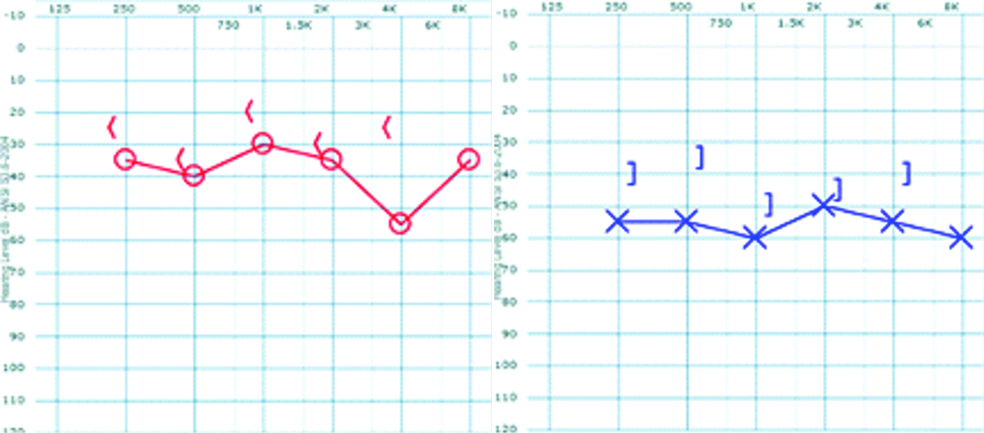
Pure tone audiometry measured for both ears revealing a mild sensorineural hearing loss in the right ear with a pure tone average of 35 dBHL and moderate sensorineural in the left ear with a pure tone average of 55 dBHL. Symbols: X and O- left and right AC thresholds respectively; <and ] -right unmasked and left masked BC thresholds respectively.
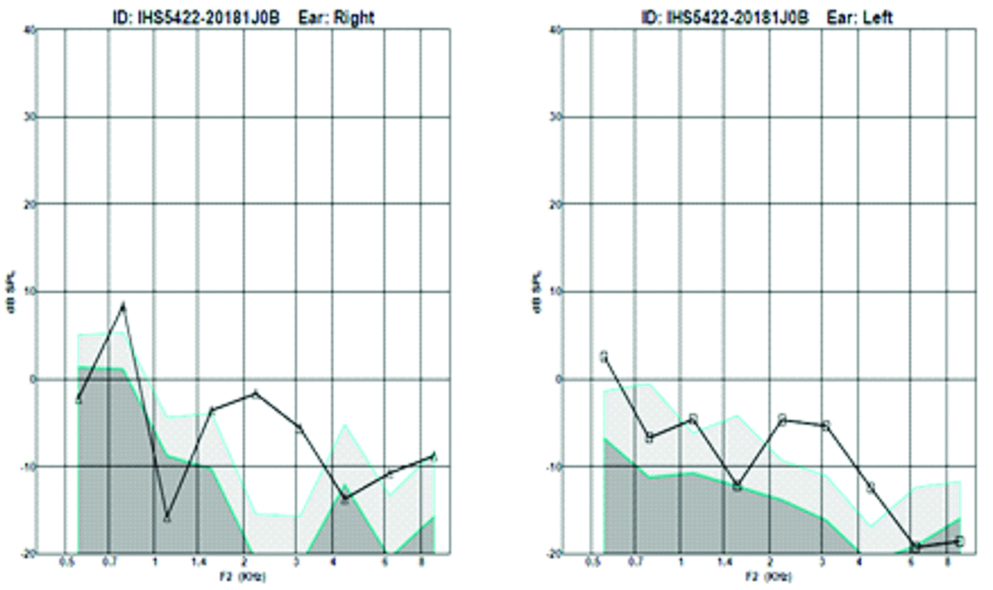
Oto acoustic emissions done in both ears revealing absent distortion product oto acoustic emissions in right ear and partially present distortion product oto acoustic emissions in the left ear.
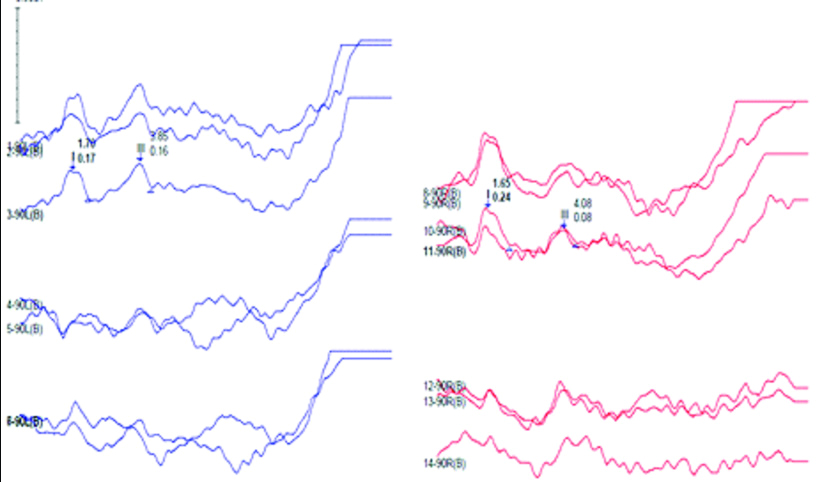
ABR waveforms in both ears. ABR done at 90dBnHL using repetition rate of 11.1/sec revealing replicable I and III peaks with absent V peak due to poor replicability and wave morphology in both ears. No ABR peaks could be obtained when using a repetition rate of 90.1/sec.
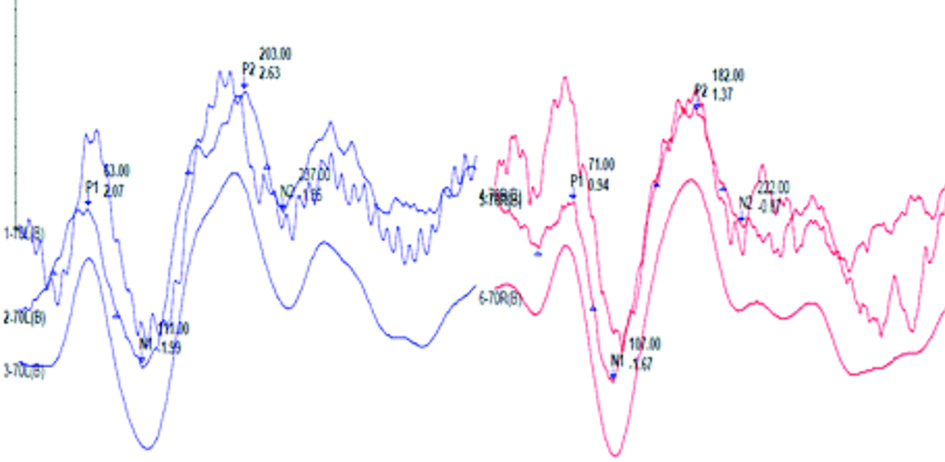
LLR waveforms obtained at 70dBnHL in both ears using speech stimuli (/da/) at a repetition rate of 1.1/sec with normal P1 N1 P2 N2 complex in both ears.
Speech evaluation was done that revealed mixed dysarthria using Franchays Dysarthria Assessment (FDA) [1]. The diagnostic formulation included slurred speech; reduce breath support for speech, reduced strength and range of articulators, affected intelligibility with distorted speech, inappropriate pitch, inappropriate loudness and mild cognitive overlay [Table/Fig-5].
Frenchay’s dysarthria assessment scoring grid for the patient. Impression reveals mixed dysarthria.
| Section | Sub test | A | B | C | D | E |
|---|
| Reflex | Cough |  |  | | | |
| Swallow |  |  | | | |
| Dribble/Drool |  | | | | |
| Respiration | At rest |  |  | | | |
| In speech |  |  |  | | |
| Lips | At rest |  | | | | |
| Spread |  |  |  |  |  |
| Seal |  |  |  | | |
| Alternate |  |  |  |  | |
| In speech |  |  |  | | |
| Jaw | At rest |  | | | | |
| In speech |  |  | | | |
| Soft palate | Fluids |  | | | | |
| Maintenance |  | | | | |
| In speech |  | | | | |
| Laryngeal | Pitch |  |  |  |  | |
| Time |  |  |  | | |
| Volume |  |  | | | |
| Tongue | At rest |  |  | | | |
| Protrusion |  |  |  | | |
| Elevation |  |  |  |  | |
| Lateral |  |  |  |  |  |
| Alternate |  |  |  | | |
| In speech |  |  |  |  | |
| Intelligibility | Words/Repetition |  |  |  | | |
| Sentences/Description |  |  |  | | |
| Conversation |  |  |  | | |
A demonstration therapy of breathing exercises, isometric and isotonic exercises to oral structures, tongue elevation, depression lateralisation exercises, jaw range of motion exercises were demonstrated and provided to the patient during initial sessions. However, he had to go back to his native, due to which he couldn’t attend regular therapy sessions. The clinicians had counseled the patient to follow the same at home and a follow-up phone call was done three months later and upon perceptual analysis a progress was reported in accordance with the AYJNIHH (AliYavar Jung National Institute of Hearing Handicapped) Speech Intelligibility rating scale [2], where the client had a score of 5 during assessment indicating a comprehension of speech with effort of speech with known context and a progressive score of 3 indicating comprehension of speech with minimal effort.
Discussion
The TBI is a temporary or permanent impairment of brain function caused by a physical injury. This can be in the form of open head injury or closed head injury. Open head injuries are where the scalp and skull are being penetrated and the dura is exposed. Closed head injuries are where the head gets struck and violently shaken causing acceleration and deceleration injury which results in coup (injury at the point of impact), countercoup (injury at the opposite pole) and diffused (injuries that is widespread) injuries [3]. TBI can be classified as: A) moderate- severe; B) mild; and C) symptomatic, based on the Mayo severity classification. The severity is based on the symptoms and scores obtained in the Glasgow Coma Scale (GCS) [4]. DAI occurs when there is a rapid acceleration and deceleration that is mainly caused by road traffic accidents, which leads to shearing of axons resulting in widespread disruption of axonal fibres. Individuals with DAI face loss of consciousness for more than six hours. Biochemical reactions take place at the level of nerve fibres that can alter the severity of the injury. Shaken baby syndrome is a typical example of a DAI [3].
Histopathological evidences suggest that DAI results in the deformation of white matter tracts and interrupts axonal transport resulting in axonal swellings. There is accumulation of APP that is visible within the first two hours of injury, excess APP results in plaque formation that is harmful. APP histopathological findings are one of the best methods to detect DAI. Normal axons are ductile and stretchable, but with the moment of head impact, the strain given to axons results in axons becoming brittle. Axonal swelling result in cytoskeleton disruption and can induce secondary axotomy, there is also breakage of axons and mitochondrial dysfunction [5]. For TBI cases, it is known that radiological test batteries aid in the diagnosis, especially an axonal injury. Various radiological studies have been documented in individuals with DAI. Diffuse Tensor Imaging (DTI) is a non-invasive imaging technique that can probe into deep tissues [6]. A study carried out by Huisman TA et al., on DTI as potential biomarker of white matter injury in DAI reveals that DTI can spot changes in white matter that correlate with both acute GCS and Rankin scores at discharge making it a valuable biomarker to detect tissue injury [7]. However, these being sophisticated test procedures, at earlier stages electrophysiological measures must be carried out. There are electrophysiological markers that can help in diagnosing TBI, some of the markers can be an alpha activity and sleep spindles that aids in diagnosis [7]. There have been documentations carried out with auditory EP being used on individuals with TBI. Vander Werff KR and Rieger B carried out click evoked and speech evoked ABR in 32 individuals with mild TBI and 32 age matched controls. They found out that there was a delay in I, III and V peak latencies in individuals with mild TBI and significant difference in amplitude and latency was noted in speech evoked ABR between experimental and control indicating a deficit subcortical processing of auditory information [8].
Kraus N et al., examined frequency following response in 20 children with a history of concussion and 20 normal children, they found out that in children with a history of concussion exhibited poor pitch coding and delayed smaller neural response, when compared to normal [9]. EPs have the capacity to detect different types of TBI and reduced amplitude and delayed latencies in EPs like the mid latency response help in identifying brainstem lesions [10]. In the present case report, electrophysiological tests revealed normal latency and morphology in lower brainstem regions, but abnormality was visualised in upper brainstem regions. Normal LLR findings could be observed but abnormal speech scores were present. With ABR taken into consideration, there could be a dysynchrony at the level of upper brainstem regions. A similar finding by Munjal SK et al., revealed prolonged V peak latencies and delayed wave I to V interpeak latencies while carrying out Binaural ABR on 190 individuals [11]. Another study by Sullivan EG et al., found a delayed III and V in peak absolute latencies, delayed interpeak latencies was also found in a patient with severe TBI [12]. With respect to late auditory EP, a study by Eskbridge EL et al., documented P300 responses in military individuals with a history of blast exposure, results revealed a delay in P300 response confirming earlier evidence of blast induced mildTBI affecting auditory and cognitive processing [13]. In terms of speech characteristics, individuals who have history of TBI tend to demonstrate slow speech rate, slow articulation rate, affected prosody with other features of dysarthria including harsh voice quality and reduced range of motions of active articulators [14,15]. These findings also reveal that upper brainstem regions are more susceptible to damage during a TBI and support the findings of present study.
Conclusion(s)
The findings reveal that electrophysiological procedures can be a better predictor in identifying diffuse lesions. Both radiological and electrophysiological investigations must be carried out in order to identify diffused lesions even though they are less severe.
Author Declaration:
Financial or Other Competing Interests: None
Was informed consent obtained from the subjects involved in the study? Yes
For any images presented appropriate consent has been obtained from the subjects. NA
Plagiarism Checking Methods: [Jain H et al.]
Plagiarism X-checker: Apr 18, 2020
Manual Googling: Jun 10, 2020
iThenticate Software: Aug 08, 2020 (10%)
[1]. Samar VJ, Metz DE, Criterion validity of speech intelligibility rating-scale procedures for the hearing-impaired populationJ Speech Hear Res 1988 31(3):307-16.10.1044/jshr.3103.3073172748 [Google Scholar] [CrossRef] [PubMed]
[2]. Enderby P, Frenchay dysarthria assessmentBr J Disord Comm 1980 15(3):165-73.10.3109/13682828009112541 [Google Scholar] [CrossRef]
[3]. Parikh S, Koch M, Narayan RK, Traumatic brain injuryInternational Anesthesiology Clinics 2007 45(3):119-35.10.1097/AIA.0b013e318078cfe717622833 [Google Scholar] [CrossRef] [PubMed]
[4]. Malec JF, Brown AW, Leibson CL, Flaada JT, Mandrekar JN, Diehl NN, The mayo classification system for traumatic brain injury severityJ Neurotrauma 2007 24(9):1417-24.10.1089/neu.2006.024517892404 [Google Scholar] [CrossRef] [PubMed]
[5]. Williamson JB, Heilman KM, Porges E, Lamb D, Porges SW, A possible mechanism for PTSD symptoms in patients with traumatic brain injury: Central autonomic network disruptionFrontiers in Neuroengineering 2013 6:1310.3389/fneng.2013.0001324391583 [Google Scholar] [CrossRef] [PubMed]
[6]. Arfanakis K, Haughton VM, Carew JD, Rogers BP, Dempsey RJ, Meyerand ME, Diffusion tensor MR imaging in diffuse axonal injuryAm J Neuroradiol 2002 23(5):794-802. [Google Scholar]
[7]. Huisman TA, Schwamm LH, Schaefer PW, Koroshetz WJ, Shetty-Alva N, Ozsunar Y, Diffusion tensor imaging as potential biomarker of white matter injury in diffuse axonal injuryAm J Neuroradiol 2004 25(3):370-76. [Google Scholar]
[8]. Vander Werff KR, Rieger B, Brainstem evoked potential indices of subcortical auditory processing following mild traumatic brain injuryEar and Hearing 2017 38(4):e20010.1097/AUD.000000000000041128319479 [Google Scholar] [CrossRef] [PubMed]
[9]. Kraus N, Thompson EC, Krizman J, Cook K, White-Schwoch T, LaBella CR, Auditory biological marker of concussion in childrenScientific Reports 2016 6:3900910.1038/srep3900928005070 [Google Scholar] [CrossRef] [PubMed]
[10]. Urakami Y, Electrophysiologic evaluation of diffuse axonal injury after traumatic brain injuryJ Neurol Neurophysiol 2013 4:15710.4172/2155-9562.1000157 [Google Scholar] [CrossRef]
[11]. Munjal SK, Panda NK, Pathak A, Relationship between severity of Traumatic Brain Injury (TBI) and extent of auditory dysfunctionBrain Injury 2010 24(3):525-32.10.3109/0269905090351687220184409 [Google Scholar] [CrossRef] [PubMed]
[12]. Sullivan EG, Guernon A, Blabas B, Herrold AA, Pape TL, Familiar auditory sensory training in chronic traumatic brain injury: A case studyDisability and rehabilitation 2018 40(8):945-51.10.1080/09638288.2016.127740328102097 [Google Scholar] [CrossRef] [PubMed]
[13]. Eskridge SL, Macera CA, Galarneau MR, Holbrook TL, Woodruff SI, Macgregor AJ, Injuries from combat explosions in Iraq: Injury type, location, and severityInjury 2012 43(10):1678-82.10.1016/j.injury.2012.05.02722769977 [Google Scholar] [CrossRef] [PubMed]
[14]. Wang YT, Kent RD, Duffy JR, Thomas JE, Dysarthria associated with traumatic brain injury: speaking rate and emphatic stressJ Commun Disord 2005 38(3):231-60.10.1016/j.jcomdis.2004.12.00115748726 [Google Scholar] [CrossRef] [PubMed]
[15]. Goozée JV, Murdoch BE, Theodoros DG, Physiological assessment of tongue function in dysarthria following traumatic brain injuryLogopedics Phoniatrics Vocology 2001 26(2):51-65.10.1080/14015430175320742111769343 [Google Scholar] [CrossRef] [PubMed]