Nephrotic syndrome without haematuria following the sequelae of the infection is uncommon. The present case report describes nephrotic syndrome following varicella infection, without haematuria and hypertension in a 21-year-old young adult. The patient presented with four weeks history of fever, swelling of both feet and facial puffiness. General physical examination revealed vesicular lesions with skin crusts present on the face, trunk and extremities, Blood Pressure (BP)-110/70 mmHg, bilateral pedal oedema. Systemic examination was unremarkable. Laboratory data showed nephrotic range proteinuria, hypoalbuminaemia. Varicella Zoster Virus (VZV) was detected in blood through Polymerase Chain Reaction (PCR). Renal biopsy confirmed the diagnosis. Thus, viral glomerulopathies should be considered in patients following infection.
Hypoalbuminaemia, Proteinuria, Viral glomerulopathies
Case Report
A 21-year-old male presented with periorbital puffiness and swelling of both feet, since four weeks. The patient had history of fever, upper respiratory tract infection associated with vesicular rash over the trunk, extremities and face six weeks ago for which he did not receive any treatment. There was no history of breathlessness, haematuria, hypertension or significant family history. There was no history of chicken pox vaccination during childhood and also drug history or history of risk behavior was also negative.
On general physical examination, the patient was afebrile, pulse was 84/min, blood pressure was 110/70 mmHg, periorbital oedema, bilateral pedal oedema, crusted vesicular lesions on the extremities, [Table/Fig-1] trunk and face. Jugular Venous Pressure was normal.
Crusted vesicular lesions on the lower limb.
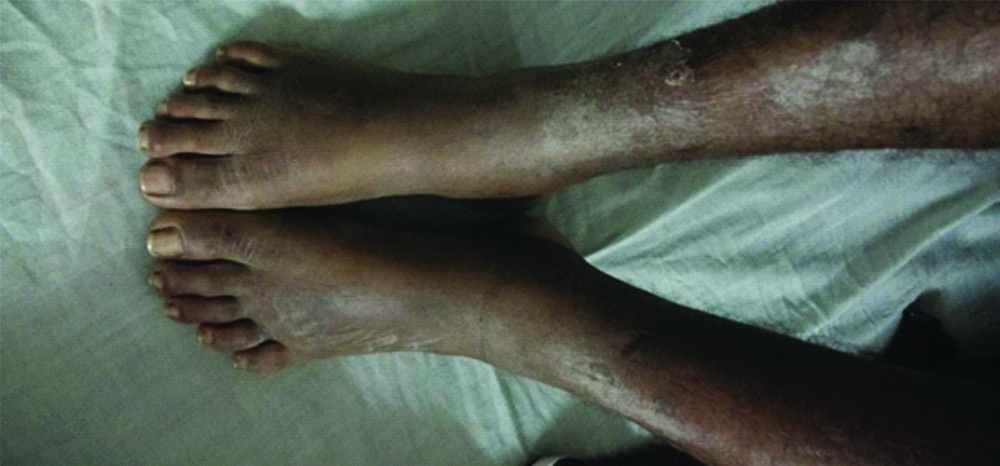
Investigations revealed Haemoglobin (Hb)- 11.4 g/dL, White Blood Cell (WBC)- 9000/cumm, Platelets- 215,000/cumm, Differential Leucocyte Count (DLC)- Neutrophils 75, Eosinophils 2, Monocytes 1, Erythrocyte Sedimentation Rate (ESR)- 48 mm/hr. Serum creatinine- 1.0 mg/dL, urea- 25 mg/dL, sodium- 135 meq/l, Potassium (K)- 4.0 meq/l, Random blood glucose 98 mg/dL, Total protein- 4.9 mg/dL and Serum Albumin was 2.8 mg/dL. Urine routine examination showed protein 3+, no Red Blood Cell (RBC)/casts or crystals. 24 hour urinary protein was 5.3 gm. Lipid profile showed total cholesterol of 208 mg/dL, Triglycerides- 424 mg/dL, Low-density Lipoprotein (LDL)- 120 mg/dL and High-density Lipoprotein (HDL)- 38 mg/dL.
Ultrasonography (USG) abdomen showed right kidney to be of 11.7×5.1 cm and left kidney having 11.7×6.3 cm dimensions, both kidneys were bulky oedematous with mild increase in cortical thickness. Electrocardiogram (ECG), plain chest radiograph and 2D Echocadiography were normal.
Antistreptolysin O (ASO) titers were negative. Antinuclear Antibody (ANA) and Anti-neutrophil cytoplasmic antibody (ANCA) were negative. Complement levels of C3 and C4 were normal. Blood PCR for VZV was positive. Renal biopsy showed segmental sclerosis at the tubular pole accompanied by foamy macrophages, podocyte capping and mild periglomerular fibrosis. There was no mesangial cells or endocapillary hypercellularity, crescent or necrosis. There was mild acute tubular injury with tubule dilatation and flattening of epithelium. Tubular epithelial cells show focal vacuolations. Interstitium appeared oedematous. These findings were suggestive of Focal segmental Glomerulosclerosis (FSGS) [Table/Fig-2].
Renal biopsy showing features of Focal Segmental Glomerulosclerosis (FSGS) (40X, H&E).
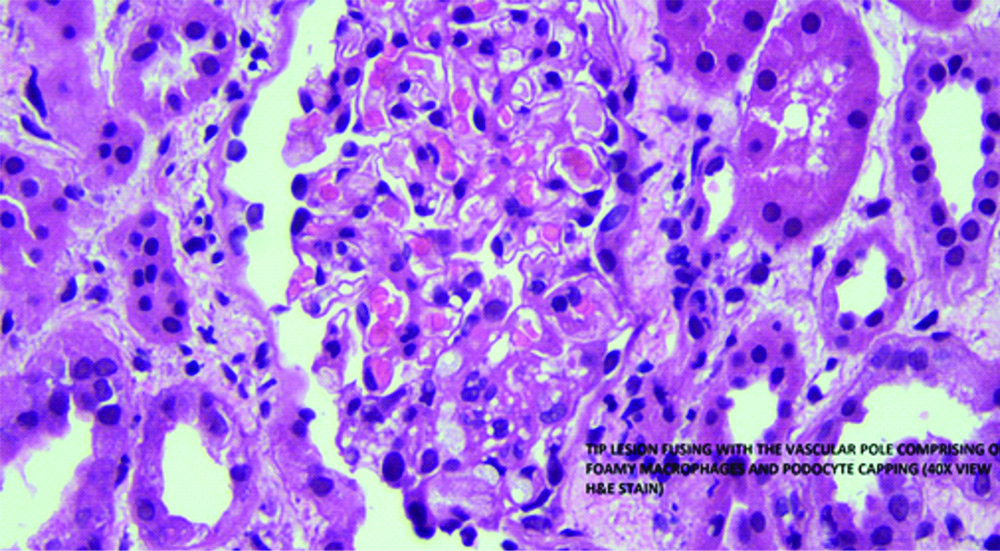
Thus, the diagnosis of nephrotic syndrome with FSGS was made following untreated varicella zoster infection. The patient was started on prednisolone 60 mg/day and was on follow-up. Subsequent follow visit, three months ago, revealed that 24 hour urine protein was 4.1 gm.
Discussion
The VZV is one of eight herpes viruses known to cause human infection. According to World Health Organisation (WHO), Global annual disesae burden of varicella is estimated to be 140 million cases. A total of 4.2 million severe complications and 4200 deaths was observed [1]. The characteristic skin lesions in all stages of development provide the basis for the clinical diagnosis of infection. It is highly contagious with an attack rate of atleast 90% among susceptible individuals during seasonal peaks. The incidence is markedly decreasing as the varicella vaccine has become more widely used [2]. But in many developing countries like India, varicella vaccination is still not included in the national immunisation programme, therefore all age groups are equally susceptible, though newer vaccines are being introduced in some states.
VZV was the first virus to be associated with nephritis, identified by Edward Heinrich in 1884 [3]. Viruses associated with minimal change nephrotic syndrome/FSGS are Adenovirus, herpes simplex, varicella zoster, Epstein barr virus, cytomegalovirus, parvovirus, Respiratory syncytial virus, influenza virus, Hepatitis A virus and Human Immunodeficiency Virus (HIV) [4].
The incubation period of VZV infection ranges from 10-21 days and the respiratory tract is the most important portal entry where it multiplies locally to initiate a silent local infection followed by haematogenous transport to cause extensive multiplications before the systemic illness is manifested. Glomerulopathy occurs by direct infection of glomerular cells causing cytopathic injury. Viruses can be antigenic stimulus for the immune system, leading to autoimmunity against cross reactive glomerular cell epitopes [5]. The clinical manifestations of the latent virus appear after prolonged periods of quiescence during which the virus remains hidden in the nerve root ganglia resulting in renal injury. The association of nephrotic syndrome in varicella, although very rare was observed in the study conducted by Kole AK et al., [6].
Unequivocal confirmation of the diagnosis is possible through the isolation of VZV in susceptible tissue cultures, demonstration of seroconversion or greater rise in antibody titres between convalescent and acute phase serum specimens or detection of VZV Deoxyribonucleic Acids (DNA) by PCR. The patient had clinical evidence of chicken pox and blood PCR for VZV was positive. There are reports of acyclovir nephrotoxicity [7], but this patient did not receive treatment soon after the onset of illness. It is difficult to link the acute onset of FSGS to acute viral infections. Direct infection of podocytes might be one of the key mechanisms in viral related FSGS [8]. Foot process effacement is reversible and may be a protective response, by podocytes secure adhesion to glomerular basement membrane and escape detachment. With improvement of local condition, podocytes resume original shape and functionality improves. Nephropathy in acute form of infection associated with FSGS is due to direct infection of glomerular cells while the chronic form of nephropathy is due to deposits of immune complexes in-situ [9]. Viral proteins induce the synthesis of mediators leading to sclerosis and worsening nephropathy [10].
Various studies [11,12] have reported the higher frequency of infections causing relapse in children with nephrotic syndrome and a prolonged course of illness resulting in progressive chronic kidney disease. This patient did not give any history of hypertension, urinary abnormalities or any other infections and no family history. To the best of author knowledge, this is the first case report of nephrotic syndrome with FSGS following untreated VZV infection as evidenced by characteristic skin lesions, positive VZV PCR and renal biopsy findings; posing a diagnostic challenge. There could be a possibility of Idiopathic Nephrotic Syndrome during the childhood which was undiagnosed in this patient. Infections are the most common cause of mortality in Idiopathic Nephrotic Syndrome. Several viral agents like Varicella Zoster, Influenza and Parainfluenza are the triggers for the disease relapse causing life threatening consequences in such patients [13].
In a study from Italy in 2015, 218 children with a first presentation of Idiopathic nephrotic syndrome, 12.4% developed infections, amongst which there was one case of primary Varicella infection [14]. In this patient, there could have been asymptomatic proteinuria for a longtime which was unnoticed as there was no history of hypertension and the patient was never evaluated in the past. The Varicella infection could be the triggering factor for the relapse which manifested as significant proteinuria, hypoalbuminaemia and dyslipidaemia.
Even though virus related FSGS is not very common, it is important to consider the possibility that the impairment of renal function in a patient may be caused by an infection. Screening for genetic mutations is required in order to differentiate between primary and secondary forms of FSGS. The prognosis of infections related FSGS is variable. Eradication of varicella is possible, if sustained vaccine coverage of at least 80% is ensured [15].
Conclusion(s)
Universal vaccination of children as a preventive measure and appropriate antiviral therapy following acute infection with follow-up in adults reduces the complications and death. It is important to be aware of the common pathogens responsible for infections in Idiopathic nephrotic syndrome for suitable treatment strategy.
Author Declaration:
Financial or Other Competing Interests: None
Was informed consent obtained from the subjects involved in the study? Yes
For any images presented appropriate consent has been obtained from the subjects. Yes
Plagiarism Checking Methods: [Jain H et al.]
Plagiarism X-checker: Mar 05, 2020
Manual Googling: Jun 12, 2020
iThenticate Software: Aug 08, 2020 (12%)
[1]. World health organisationVaricella and herpes zostervaccines: WHO position paperWeekly Epidemiological Record 2014 89(25):265-88. [Google Scholar]
[2]. Marin M, Marti M, Kambhampati A, Jeram SM, Seward JF, Global varicella vaccine effectiveness: a meta-analysisPaediatrics 2016 137(3):e20153741310.1542/peds.2015-374126908671 [Google Scholar] [CrossRef] [PubMed]
[3]. Reimann HA, Nephropathy and virusesPostgrad Med J 1968 44:853-60.10.1136/pgmj.44.517.8534303136 [Google Scholar] [CrossRef] [PubMed]
[4]. Wenderfer SE, Viral glomerulopathies in childrenPaediatr Nephrol 2015 30(11):1929-38.10.1007/s00467-015-3057-y25752759 [Google Scholar] [CrossRef] [PubMed]
[5]. MacKay IR, Science, medicine and the future: Tolerance and autoimmunityBMJ 2000 321:93-96.10.1136/bmj.321.7253.9310884262 [Google Scholar] [CrossRef] [PubMed]
[6]. Kole AK, Roy R, Kole DC, An observational study of complications in chickenpox with special reference to unusual complications in an apex infectious disease hospital, Kolkata, IndiaJ Postgrad Med 2013 59:93-97.10.4103/0022-3859.11381123793307 [Google Scholar] [CrossRef] [PubMed]
[7]. Chavez-Iniguez JS, Medina-Gonzalez R, Aguilar-Parra L, Torres-Vázquez EJ, Maggiani-Aguilera P, Cervantes-Pérez E, Oral acyclovir induced hypokalemia and acute tubular necrosis a case reportBMC Nephrol 2018 19:32410.1186/s12882-018-1121-030428841 [Google Scholar] [CrossRef] [PubMed]
[8]. Dettmar AK, Oh J, Infection related Focal segmental Glomerulosclerosis in childrenBio Med Res Int 2016 2016:735196410.1155/2016/735196427294131 [Google Scholar] [CrossRef] [PubMed]
[9]. Lai A, Lai KN, Viral nephropathyNature Clinical Practice nephrology 2006 2(5):254-62.10.1038/ncpneph016616932438 [Google Scholar] [CrossRef] [PubMed]
[10]. Segerer S, Nelson PJ, Chemikines SD, Chemokine receptors and renal disease: From basic science to pathophysiological and therapeutic studiesJ Am Soc Nephrol 2000 11(1):152-76.10.1681/ASN.V11115210616852 [Google Scholar] [CrossRef] [PubMed]
[11]. Alwadi RK, Mathew JL, Rath B, Clinical profile of children with nephrotic syndrome not on glucocorticoid therapy, but presenting with infectionJ Paediatr Child Health 2004 40:28-32.10.1111/j.1440-1754.2004.00285.x14718000 [Google Scholar] [CrossRef] [PubMed]
[12]. Moorani KCN, Infections are common cause of relapse in children with nephrotic syndromePak Paed J 2011 35(4):213-19. [Google Scholar]
[13]. McCaffrey J, Lennon R, Webb NJA, The non suppressive management of childhood nephrotic syndromePediatr Nephrol 2016 31:1382-402.10.1007/s00467-015-3241-026556028 [Google Scholar] [CrossRef] [PubMed]
[14]. Pasini A, Aceto G, Ammenti A, Ardissino G, Azzolina V, Bettinelli A, Best practice guidelines for Idiopathic nephrotic syndrome: recommendations versus realityPediatr Nephrol 2015 30:91-101.10.1007/s00467-014-2903-725127916 [Google Scholar] [CrossRef] [PubMed]
[15]. Dehal N, Krishnan K, Kanchan T, Singh J, Public funded immunisation: key to varicella control in IndiaThe Lancet 2015 386:2389-23.10.1016/S0140-6736(15)01190-3 [Google Scholar] [CrossRef]