VAP is defined as the new onset of parenchymal lung infection, 48 hours after endotracheal intubation and/or mechanical ventilation [1]. VAP is an important and issue of concern in the Intensive Care Unit (ICU) due to prolonged mechanical ventilation, prolonged ICU stay, high health care expenditure and high risk of death [2,3]. In spite of availability of effective antimicrobial regimen, the mortality rate of VAP is still high, ranging from 33 to 50% [1]. The response to treatment of VAP and prognosis of patients should not depend on a single parameter, therefore there were many scores and markers used such as Acute Physiology And Chronic Health Evaluation (APACHE II) score, Sequential Organ Failure Assessment (SOFA) score, CPIS, leukocytic count, CRP and procalcitonin (PCT) [4]. Biomarkers like PCT, CRP, mid-region fragment of pro-adrenomedullin (MR-proADM), triggering receptor expressed on myeloid cells 1 (TREM 1), arterial natriuretic peptide, Copeptin, endocan are used to diagnose VAP rapidly in different studies [5-7]. Inflammatory markers such as CRP are still widely used in the diagnosis of VAP, although they have low sensitivity in ICU setting [1,8]. Some observational studies showed that CRP value does not make any difference among survival and non-survival of VAP patients [9-12]. Whereas other studies found serial measurements of CRP quite useful to predict outcome of VAP [12,13].
CRP can be used as a marker to know the response to antibiotics in VAP patients [13,14]. As the role of CRP in the prognosis of VAP is inconclusive, further in-depth study is essential for evaluation. Value of serial measurements of CRP in the assessment of VAP, after initiation of antibiotic therapy or survival of patients was limited.
As there is lack of sufficient Indian data regarding the prognostic implication of CRP in VAP. So the objective of this study was to assess the prognostic value of progressive CRP levels in patients with VAP and compare with non-VAP group of ventilated patients.
Materials and Methods
This prospective, observational cohort study was conducted between November 2017 and October 2018 at Central Intensive Care Unit (CICU). The CICU is comprised of 20 beds and patients were either admitted through emergency department or transferred from Medical ward (primary medical cause) or surgical ward (primary surgical cause). The study was approved by the Institutional Ethics Committee (letter no-2015/P-1-RP/133) and an informed consent was taken from the legally authorised representative of the patients. The privacy and confidentiality of study participants and data were maintained during all stages of this study.
Inclusion criteria: Patients on mechanical ventilator for more than 48 hours due to any cause were included in the study.
Exclusion criteria: The patients, those having pre-existing Pneumonia before intubation; or pneumonia within 48 hours of intubation; pre-existing lung disease (clinically/radiologically); age less than 18 years; or those patients could not be followed (left against medical advice) were excluded from this study.
Simple Consecutive sampling technique was applied during the study. Sample size estimation was done by consideration of power 80% and one side alpha error 2.5% through comparison of proportion based on previous study i.e., on day 4 of antibiotic therapy, the value of CRP decreased to 47% of the initial value in survival group while it was 96% in nonsurvivors group of VAP patients [15]. Minimum sample size was 12 in each group (survivor group and non-survival group). Study subjects were divided into two cohorts (VAP group and non-VAP group).
Data was collected through structured format (case report format) which contained information like primary diagnosis, demographic data, vital signs, APACHE II. Some clinical data like tracheal secretions, X-ray chest, temperature in °C, leukocyte count, PaO2/FiO2, SOFA score were recorded daily during morning round. The diagnosis of VAP was made in study subjects having Modified CPIS>6 along with performing a quantitative culture of the endotracheal aspirate/bronchoalveolar lavage/protected specimen brush and observing ≥105 CFU/mL isolates with infiltration or consolidation in Chest X-ray.
Culture of respiratory sample and X-ray chest was done after suspicion of VAP i.e., CPIS score >6. All VAP patients received empirical antibiotic therapy as per institutional protocol. Antibiotics were modified based on culture sensitivity report. Early onset VAP means diagnosis of VAP within 5 days of mechanical ventilation and late VAP means diagnosis of VAP 5 days or later of mechanical ventilation.
[Table/Fig-1] shows scoring system of modified CPIS based on clinical, radiological, haematological and microbiological parameters. The study subjects were monitored from 3rd, 4th, 5th, 6th, 7th, 8th, 9th and 16th day of ICU stay for the development of VAP, using clinical and laboratory criteria term as D1, D2, D3, D4, D5, D6, D7 and D14 observation day, respectively. Blood samples were obtained via venous line on admission and subsequently during every morning round. Quantitative CRP measurements were done daily for 7 consecutive days and on day 14 of observation day in those who survived and were on ventilator in study subjects. Quantitative CRP measurements were done using semi-automated clinical chemistry analyser (AT-200D, Accurex Biochemical Pvt., Ltd., Mumbai, India) through Immuno-turbidometric method. End point of study was either death of patient or withdrawal from mechanical ventilator. The evolution of CRP concentration throughout the course of VAP and non-VAP were analysed and compared between survivors and non-survivors.
Modified Clinical Pulmonary Infection Score (CPIS) score.
| CPIS points | 0 | 1 | 2 |
|---|
| Tracheal secretions | Rare | Abundant | Abundant and purulent |
| Chest X-ray infiltrates | No infiltrate | Diffuse | Localised |
| Temperature (°C) | ≥36.5 and ⊄38.4 | ≥38.5 and ⊄38.9 | ≥39 or ⊄36 |
| Leukocyte count (mm3) | >4,000 and <11,000 | <4,000 and >11,000 | <4,000 or >11,000 and band forms |
| PaO2/FiO2 (mmHg) | >240 or ARDS | - | ⊄240 and no ARDS |
| Culture of tracheal aspirate | Negative | -- | Positive |
Statistical Analysis
The statistical analysis was performed with the help of Statistical Package for the Social Sciences (SPSS) (Version 22, IBM). Continuous variables were presented as mean (±SD). Categorical variables were expressed as percentage. Comparison of different mean was done with Student’s t-test and comparison of proportions was done with the chi-square test. The p-value of 0.05 or less was considered statistically significant. Time-dependent analysis of mean CRP was performed via excel sheet.
Results
In this study, 65 subjects were included. Out of which, 27 were diagnosed as VAP and rest 38 subjects were non-VAP. [Table/Fig-2] shows the base line characteristics of ventilated patients. Majority (62%) of patients were male, out of which 23 were in non-VAP group. The majority (75%) of patients were from medical ward and the rest from surgical ward. Age, gender and the primary type of patient (either medical ward or surgical ward), number of ventilator days, baseline CPIS score, SOFA score and APACHE II score (Day-1) were matched in both (VAP/non-VAP) group as p-value >0.05 (as shown in [Table/Fig-2]). Out of 27 patients, early VAP was seen in 13 patients and late VAP in 14 patients. There were 12 deaths in VAP group and 14 deaths in non-VAP group.
Demographic characteristics between patient with Ventilator Associated Pneumonia (VAP) and non Ventilator-Associated Pneumonia (VAP).
| Characteristics | Non-VAP (n-38) | VAP (n-27) | p-value |
|---|
| Age (years): Mean±SD | 48.29±17.53 | 46.22±19.60 | 0.65 |
| Male | 23 | 17 | 0.52 |
| Primary type of patient (Medical) | 29 | 20 | 0.53 |
| Ventilator days (Mean±SD) | 7.87±3.46 | 9.37±3.44 | 0.09 |
| Baseline CRP | 10.02±4.18 | 9.19±3.68 | 0.41 |
| Baseline CPIS score | 3.37±0.883 | 3.63±0.68 | 0.20 |
| Baseline SOFA | 6.82±1.64 | 6.52±1.47 | 0.45 |
| Baseline APACHE II score | 27.71±5.21 | 20.81±5.21 | 0.49 |
SD: Standard deviation; CRP: C-reactive protein; CPIS: Clinical pulmonary infection score; APACHE II: Acute physiology and chronic health evaluation score; SOFA: Sequential organ failure assessment score
[Table/Fig-3] shows, baseline mean value of APACHE II and SOFA score were more in non-survivor group of patients in VAP and non-VAP patients which were statistically significant. But baseline CRP and baseline CPIS score was not statistically different among survivor and non-survivor group of both cohorts.
Comparison of different baseline parameter between survival and nonsurvival among VAP and Non-VAP group of patients.
| Variable at the time of intubation | Non-VAP (n=38) | VAP (n=27) |
|---|
| Survival (n=24) | Nonsurvival (n=14) | p-value | Survival (n=15) | Nonsurvival (n=12) | p-value |
|---|
| Baseline CRP | 6.90±9.03 | 15.38±19.46 | 0.07 | 9.07±8.37 | 9.35±9.43 | 0.93 |
| APACHE II | 18.75±3.33 | 26.79±3.68 | <0.001 | 17.40±2.69 | 25.08±4.37 | <0.001 |
| Baseline CPIS | 3.25±0.84 | 3.07±0.73 | 0.51 | 4.61±0.48 | 4.33±0.65 | 0.14 |
| Baseline SOFA | 5.96±1.16 | 8.29±1.26 | <0.001 | 5.67±1.17 | 7.58±1.08 | <0.001 |
*p <0.05 was considered as statistically significant; CRP: C-reactive protein; CPIS: Clinical pulmonary infection score; APACHE II: Acute physiology and chronic health evaluation score: SOFA- Sequential organ failure assessment score
In this study, majority {10 (37%)} of VAP was caused by Klebsiella Pneumoniae. Other bacterial causes of VAP were Acinetobacterbaumanii in 7(26%), Pseudomonas aeruginosa in 4(15%), Staphylococcus aureus in 3 (11%), Escherichia coli in 2 (7%) and Stenotrophomonasmaltophilia in 1 (4%). In this study, 96% (26) of patient’s harboured ESBL isolates, 78% (21) were MDR organisms, 18% (5) were carbapenem resistant, 4% (1) were XDR organisms and all Staphylococcus aureus isolates were MRSA.
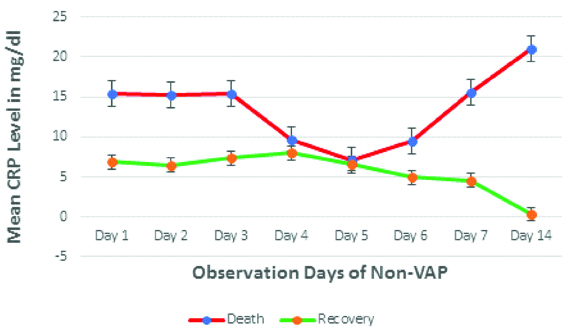
[Table/Fig-4] shows, there is decreasing trend of mean CRP level in recovered patient after 4th day onwards whereas increasing trend in non-survival patient 5th day onwards in non-VAP group.
Time trend analysis of CRP among Non-VAP patients.
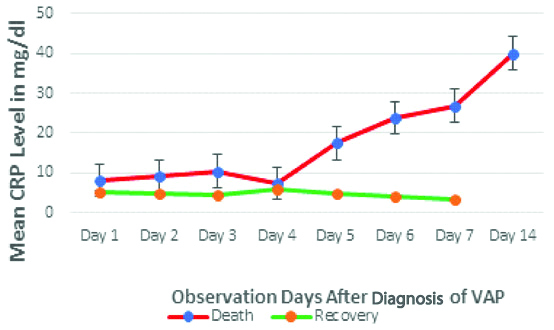
Time trend analysis of mean CRP level [Table/Fig-5], from D1 to D14 of VAP patients, showed almost no change in recovered patients whereas, in non-survivors, these parameters showed increasing trend after day 4.
Time trend analysis of CRP among VAP patients.
Discussion
In this study, VAP was diagnosed based on clinical, radiological and microbiological evidence of micro-organisms. All patients were on empiric antibiotics after suspicion of VAP i.e., CPIS score >6. The demographic data of both VAP and non-VAP group are comparable. Difference of baseline mean CRP of both VAP and non-VAP group patients and non-survivors and survivors group were not statistically significant, thus base line CRP value didn’t help much to predict VAP nor predict the survival of the ventilated patient (both VAP and non-VAP). The mean CRP showed an increasing trend in non-survivor population on day 4th onwards both in VAP and non-VAP group but this trend was not seen in recovered patients. And the difference in trend of mean CRP among non-survivor and survivor patients were more marked in VAP group patients than non-VAP group patients.
Similar finding seen by Povoa P et al., and stated that CRP kinetics was significantly different between survival and non-survival of VAP patients which can be used as outcome indicator as soon as 4th day of initiation of antibiotics [15]. A prospective study showed that, decreasing level of CRP and PCT after day 4 is an independent predictor of survival in VAP [16]. But contrary to this study finding, other studies found that there was no statistical difference in CRP value in VAP patients among survivor and non-survivor group patients and also no correlation between mortality and CRP value [10,17]. Study by Zhydkov A et al., showed that there was no significant association of baseline CRP and outcome of Community Acquired Pneumonia (CAP). But when kinetics of CRP was taken into consideration, on days 5 and 7 of CAP, it showed good prediction of both survival and adverse clinical outcomes [18]. Meisner M et al., reported that there was no statistically significant difference between survivor and non-survivor VAP patients in terms of mean serum CRP level at ICU admission [19]. Seligman R et al., CRP at the onset of VAP and 4th day of VAP can predict the survival of VAP. Decreasing value of CRP showed seven-fold greater chance of survival in VAP patients [13]. Studies stated that, CRP kinetics can identify poor outcome as soon as 4th day of initiation of antibiotics in VAP patient [13-15]. Another study pointed out that, SOFA score and PCT kinetics can be used as prognostic marker but not by CRP [11].
CRP is an acute phase inflammatory mediator whose level increases in both infective as well as inflammatory condition. Severity of increasing level of CRP co-related with the severity of lung infection or inflammation. Changes in systemic biological markers like CRP levels may indicate modification in clinical status. Though VAP arises due to infection but the condition worsens due to more inflammation. Increase inflammation may be the cause behind the increasing trend of serial CRP, also seen in non-survivor population, even in non-VAP group. In this study, mean value of base line APACHE II score and SOFA score were higher in non-survivor group than the survivor groups. So higher value of these scores were poor prognostic factors. But to get a complete value of APACHE II score or SOFA score, lot of investigations are needed, which becomes more expensive. Clinicians can get same type of information through CRP, which is a rapid, accurate and inexpensive tool. Base line CRP at the time of diagnosis of VAP may not be good indicator to know the prognosis but kinetics of CRP is probably a good indicator to predict mortality as early as 4th day after diagnosis. On the other hand, based on this study findings, serial daily CRP measurements could be used as a marker of VAP resolution which might be of some help to clinician for the reassessment of patients that fail to improve and the antimicrobial therapy.
Limitation(s)
This was a cohort single centre observational study having small sample size which limits the strength of the conclusion, where results should be verified by large multicentric study. The study population could be heterogenous, as patients from both medical and surgical ward taken into the study. Co-morbidities were not taken into account during data collection which could be confounding factor.
Conclusion(s)
From the present study, it can be concluded that serial CRP concentration is a useful tool to predict mortality, as early as 4th observation day in VAP patients and 5th observation day in non-VAP patients. Further multi-centric studies with large sample sizes are necessary to establish this fact.
Declaration: This study was presented in Congress of European Respiratory Society, which was held on Madrid, Spain on 30th September 2019.
SD: Standard deviation; CRP: C-reactive protein; CPIS: Clinical pulmonary infection score; APACHE II: Acute physiology and chronic health evaluation score; SOFA: Sequential organ failure assessment score
*p <0.05 was considered as statistically significant; CRP: C-reactive protein; CPIS: Clinical pulmonary infection score; APACHE II: Acute physiology and chronic health evaluation score: SOFA- Sequential organ failure assessment score