The ASDs are characterised by impaired social interactions, language deficit, and stereotype behaviour [1]. In Egypt, the prevalence of ASDs among children was documented to be 33.6% [2]. Frequent reports of both psychiatric and medical problems are mentioned, including social anxiety disorder, attention deficit, immune system abnormalities and mitochondrial dysfunction [3]. Due to these impairments, the quality of life of both the autism patients and the caregivers is disturbed. Treatment of autism adds a burden to society economically and socially [4].
Autism has a multifaceted nature; therefore many studies were done to understand its pathophysiology and the underlying biological mechanisms. Alterations in serotonergic systems have been reported in autism [5]. There is an increasing role of chronic inflammation in neurological disorders. Its role is not limited to specific neurotransmitter abnormalities; it produces changes in the neurotransmitter circuits, immune, endocrine and oxidative/anti-oxidant status in the brain [6].
The valproic acid model furnishes a useful model to investigate the neurobiology of autistic behaviour and to search for new therapeutics [7]. PND14 is a sensitive period during which the neuronal migration, differentiation, myelination, synaptogenesis and gliogenesis occur in the cerebellum, striatum and hippocampus [8]. VPA administration on PND14 in rodents is documented to cause intrusions and neurodevelopmental regressions that lead to behavioural retardations [9].
Ginkgo biloba extract is used frequently in many medicine recipes. Its leaf extract consists of terpenoids and flavonoids which have anti-oxidant potency [10]. It can remove oxygen free radicals, inhibit lipid peroxidation, inflammation and allergic reaction, modulate immune responses, promote cell proliferation and apoptosis [11]. Also, it improves blood flow properties, protects against ischemia and hypoxia in the brain, improves nerve cell energy metabolism and has myelin protecting effects [12]. Moreover, Ginkgo biloba leaves are prescribed as an alternative herbal medicine for memory improvement and dementia [11].
These data initiate the interest to study the neuroprotective effects of Ginkgo biloba extract on ASD induced by valproic acid in mice based on behavioural, biochemical, histological and immunohistochemical studies.
Materials and Methods
The experimental animal study was conducted in the Faculty of Medicine, Menoufia University, Egypt from the 1st of December 2019 to the end of January 2020. The sample size was calculated at 80% power with a significance level of 5% [13]. Twenty-four male Wister albino mice were used. They were fed standard laboratory chow and allowed free access to water in an air-conditioned room with a 12 hour light-dark cycle. Animal care and use were approved by the Menoufia University, Faculty of Medicine Ethical Committee (No. 12/2019 ANAT 3). After acclimatisation, mice were divided randomly into four groups (six mice/group).
The control group received an intraperitoneal injection of 100 mL saline once daily from PND13 to PND 40. Ginkgo biloba extract-treated group (GK): Received Gingko biloba extract (BLP Pharmaceuticals and Chemicals Ltd., China) via intraperitoneal injection at a dose 100 mg/kg [14] from PND13 to PND40. Valproic acid-treated group (VPA): Received Sodium Valproic acid (Sigma Aldrich, St Louis, MO, USA) at a dose 400 mg/kg/once via subcutaneous injection [15] on PND14 followed by saline thereafter up to PND 40. Valproic acid + Ginkgo biloba extract-treated group (VPA and GK): Received VPA on PND14 and Ginkgo biloba extract from PND13 to PND40 by the same doses and routes mentioned before.
At the end of the study, all mice were assessed by behavioural tests, serum biochemical study then they were sacrificed by cervical decapitation, followed by dissection of the brain. The right half of the cerebellum was fixed in 10% buffered formalin for histopathological and immunohistochemical studies while the left half was utilised for tissue biochemical analysis.
Neurobehavioural Tests
Mice were allowed to acclimatise to the behaviour laboratory for 30 minutes before testing. Tests were conducted during the daytime (9 AM–4 PM), and each test was conducted at the same time of the day. The researchers followed behaviour in real-time, with a stopwatch. Test chambers were cleaned with 70% ethanol among subjects.
Open field test: Mice were placed for five minutes inside a Plexiglas arena (30×20 cm), divided into 12 squares of equal size. The number of crossing slots, rearing movements and times of center crossing were recorded [16].
T-Maze Spontaneous Alternation: This test measures exploratory behaviour that depends on working memory. Mice were put on the base of T-maze are were allowed to move towards right or left arm for 10 consecutive trials. It was counted as an entry when all the four paws were into an arm [17].
Social Approach (Three-Chamber) Test: The test was performed in two sessions (10 minutes/each). In session one, a Plexiglass box was divided into three interconnected chambers and a mouse was placed in the central chamber. After five minutes of habituation, it was given the choice to interact with either an empty wire cup located in one side chamber or a similar wire cup with an unfamiliar mouse (stranger I) located in the opposite chamber, which was matched in strain, age and sex. Time spent interacting with each cup was measured. In session two, place a second control mouse (stranger II) which was matched in strain, age and sex inside an identical wire containment cup in the opposite side chamber. The time spent interacting with each cup was measured [18].
Blood Sample Collection
Blood was drawn via cardiac puncture then allowed to coagulate for 30 minutes at Room Temperature (RT). Blood samples were centrifuged at 2000 rpm for 10 minutes to separate serum samples. Serum samples were stored at -20°C for the assessment of IL-17 (Quantikine® ELISA, R&D Systems Inc., MN, USA) according to the manufacturer’s instructions.
Tissue Homogenate for Biochemical Analysis
All brain tissues were homogenised (10% w/v) in 10 mM of ice-cold Phosphate Buffer Saline (PBS), pH 7.4. The homogenate was centrifuged at 4000 rpm for 15 minutes at 4°C. The pellet was discarded, and the whole supernatant was used for assessment of IL-6 ELISA kit (MyBiosource, Inc, USA), GSH and MDA (QuantiChrom™, BioAssay Systems, USA) and TGF-β1 ELISA kit (R&D Systems, Minneapolis, MN) using manufacturer’s protocol.
Histopathological, Immunohistochemical and Quantitative Assessment
The cerebellum was fixed in a 10% buffered formalin solution (pH 7.4) and embedded in paraffin wax. Histopathological examination was done on 5-μm sections which were deparaffinised. These were then rehydrated with graded ethanol (100%, 90%, 70%) in a series. For staining, Hematoxylin and Eosin stain and Toluidine blue were used.
For immunohistological staining, 5-μm sections were rinsed with PBS and blocked for 30 minutes in 0.1% H2O2, as an inhibitor of endogenous peroxidase activity. After rinsing in PBS, the sections were incubated for 60 minutes in blocking solution (10% normal goat serum) at RT. The sections then were incubated with the primary antibodies {Anti-MBP, rabbit polyclonal,1: 300, Abcam, Cambridge, MA, USA, ab40389) and anti-Serotonin, goat polyclonal,1:400, ab66047, Abcam} at RT for an hour. The sections were rinsed with PBS, followed by 20 minutes of incubation at RT with the secondary biotinylated goat anti-rabbit antibody (1:200, Vector labs, Peterborough, UK, BA-1000). The sections were rinsed with PBS, followed by 20 minutes of incubation at RT with the secondary biotinylated goat anti-rabbit antibody (1:200, Vector labs, Peterborough, UK, BA-1000). After washing the sections in PBS, Streptavidin-Horseradish peroxidase solution was added to the sections for 10 minutes. A 3, 3-diaminobenzoic acid (DAB) dissolved in PBS was used to visualise the secondary antibody binding and instantly before use, H2O2 was added to a concentration of 0.03%. The tissues were rinsed with PBS then were counterstained with aqueous haematoxylin (Biomed) as recommended. The slides were washed in distilled water until the sections turned blue. Finally, the tissues were dehydrated in ascending grades of ethanol (70%, 95%, and 100%) for five minutes each, cleared in xylene, then mounting with Histomount and a coverslip was done.
For quantitative assessment, five different sections were analysed (400 X) from at least five different mice from each group to count the number of PCs, the colour intensity of toluidine blue staining of the PCs, the area percentage of MBP and serotonin using Image J software (1.74v).
Statistical Analysis
The results were expressed as mean±SD. The results were computed statistically using Statistical Package for the Social Sciences (SPSS) (version 14.0; SPSS Inc., Chicago, Illinois, USA). ANOVA was used followed by a post-hoc Tukey test. A p-value <0.05 was considered statistically significant.
Results
There was an insignificant difference between control and GK groups in all the outcomes in the study; therefore, they were pooled in one group (control).
I- Open Field Test
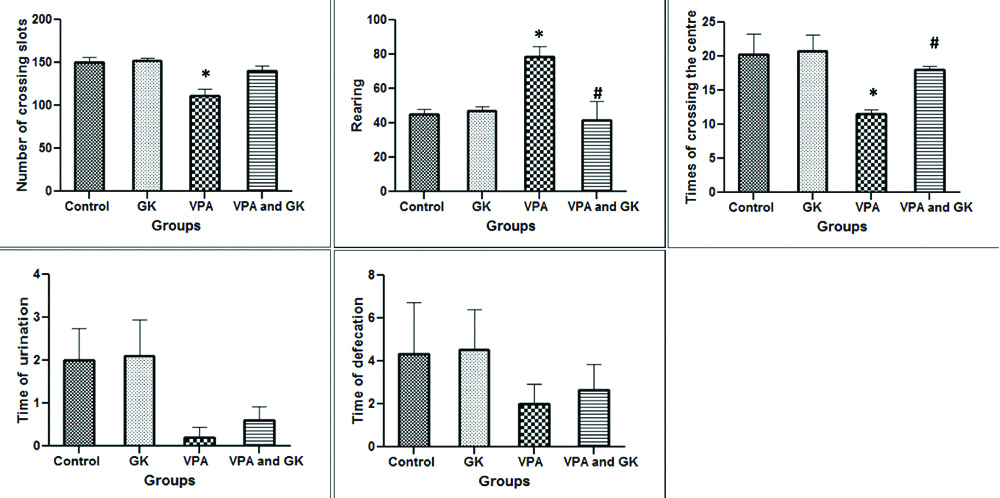
The study of open field test showed that the number of crossed slots by mice was significantly lower (p<0.05) in VPA group when compared to control (111.50±8.09 vs. 149.67±6.88). However, in VPA+GK group, the number of crossed slots (140±5.77) was insignificantly different (p>0.05) when compared to VPA and control groups. The number of rearing movement was significantly higher in VPA group when compared to the control group (78.75 ±5.89 vs. 45±2.88). On the other hand, in VPA+GK group, the number of rearing movement (41.66±10.68) was significantly lower when compared to VPA group. The times of centre crossing by mice in VPA group were significantly lower when compared to the control group (11.50±0.64 vs. 20.33±2.96). While, in VPA+GK group, the number of the times of centre crossing (18±0.57) was significantly higher when compared to VPA group. The times of urination and defecation showed insignificant difference among the studied groups [Table/Fig-1].
Open field results in the studied groups showing the number of crossing slots, rearing, times of crossing the centre, times of urination and defecation.
*Significant (p<0.05) when compared to the control group; #Significant when compared to the VPA- group. The number of mice=6/group; GK: Ginkgo biloba; VPA: Valproic acid-treated group
II- Social Approach (Three-Chamber) Test
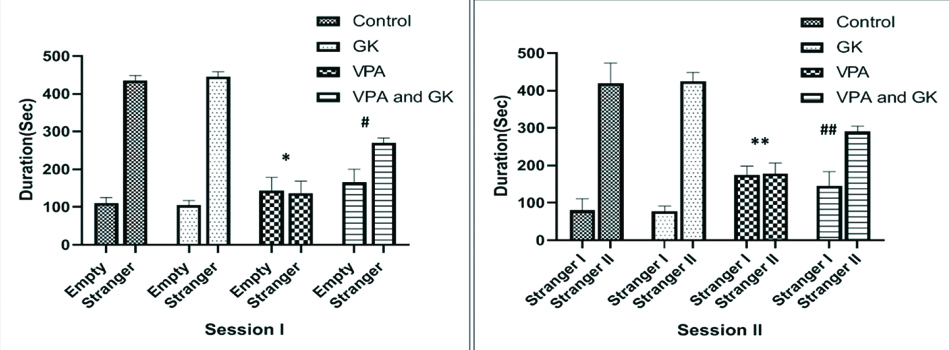
Session one demonstrated that, in the control group, the time spent by the subject mice with stranger mice was significantly higher (p<0.05) when compared to the time spent by the subject mice in the empty chamber (435±13.4 vs. 110.15.4 seconds, respectively). However, in VPA group; mice failed to demonstrate a preference for social proximity by spending nearly the same time in both empty and stranger chambers (144±34.2 vs. 136.5±32.3 seconds, respectively). In VPA+GK group, there was a significant increase (p<0.05) in the time spent with the stranger mouse in relation to the time spent in the empty chamber (270±13.4 vs. 165±35.5 seconds, respectively).
In session two; in the control group, the time spent with stranger II by the subject mice was significantly higher when compared to the time spent with stranger I (420±53.7 vs. 80±30.9 seconds, respectively). However, in VPA group; mice failed to demonstrate a preference for social proximity by spending nearly the same time in both strangers I and II chambers (175±23.3 vs. 178.7±17.8 seconds, respectively). In VPA+GK group, there was a significant increase in the time spent with stranger II in relation to the time spent in stranger I chamber (290±15.4 vs. 145±38.7 seconds, respectively) [Table/Fig-2].
Social approach (three-chamber) test results in the studied groups.
*Significant (p<0.05) when compared to the control group, **highly significant (p<0.001) when compared to the control group, #significant when compared to the VPA- group, ##highly significant when compared to the VPA-group. Number of mice=6/group; GK: Ginkgo biloba; VPA: Valproic acid-treated group
III-T-Maze Alteration Test
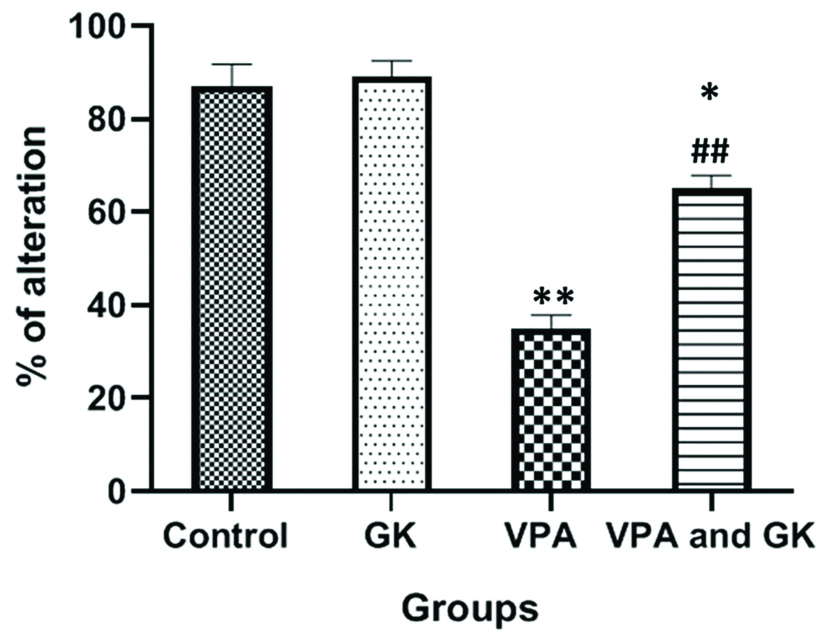
The study of the T-maze alteration test showed that; the percentage of alteration in VPA group was significantly lower (p<0.05) when compared to the control group (35%±2.88 vs 87%±4.7, respectively). In VPA+GK group, the percentage of alteration (65%±2.88) was significantly higher when compared to VPA group but still significantly lower when compared with the control group [Table/Fig-3].
T-Maze alteration test results in the studied groups.
*Significant (p<0.05) when compared to the control group, **highly significant (p<0.001) when compared to the control group, ##highly significant when compared to the VPA-group. The number of mice=6/group; GK: Ginkgo biloba; VPA: Valproic acid-treated group
IV-Biochemical Results
The brain MDA was significantly higher while GSH was significantly lower in VPA group when compared to the control group. However, their levels were significantly improved in VPA+GK group when compared to VPA group. The brain IL-6 was significantly higher in VPA group when compared to the control group. However, the IL-6 level in VPA+GK group was significantly lower when compared to VPA group. Brain TGF-β1, was significantly lower in VPA group when compared to the control group. TGF-β1 level in VPA+GK group was significantly higher when compared to VPA group. The serum level of IL-17 was significantly higher in VPA group when compared to the control group. However, IL-17 level in the VPA+GK group was significantly lower when compared to VPA group [Table/Fig-4].
Oxidative and inflammatory markers among the studied groups.
| ParametersGroups | Control group | GK group | VPA group | VPA and GK group |
|---|
| - Brain MDA (mmol/g) Mean±SD | 30.09±1.21 | 29.89±1.43 | 58.65±1.12** | 40.22±0.96*## |
| - Brain GSH (mg/g) Mean±SD | 80.26±1.28 | 81.45±1.23 | 50.40±0.29* | 61.95±1.81**## |
| - Brain IL-6 (pg/g) Mean±SD | 108.63±5.58 | 108.1±2.34 | 139.12±5.08* | 122.65±2.22# |
| - Brain TGF-β1 (pg/g) Mean±SD | 162.47±6.44 | 160.98±1.34 | 103.22±1.72** | 140±5.59*# |
| - Serum IL-17 (pg/mL) Mean±SD | 41±4.79 | 40.23±4.33 | 107.33±6.35** | 78±1.64*# |
*Significant (p<0.05) when compared to the control group; **highly significant (p<0.001) when compared to the control group; #significant when compared to the VPA-group; ##highly significant when compared to the VPA-group. Number of mice=6/group; SD: Standard deviation; GK: Ginkgo biloba; VPA: Valproic acid-treated group; MDA: Brain tissue malondialdehyde (MDA); GSH: Glutathione; IL- 6= Interleukin-6; TGF-β1- Transforming Growth Factor-Beta IL-17- serum interleukin-17
V- Histopathological Results
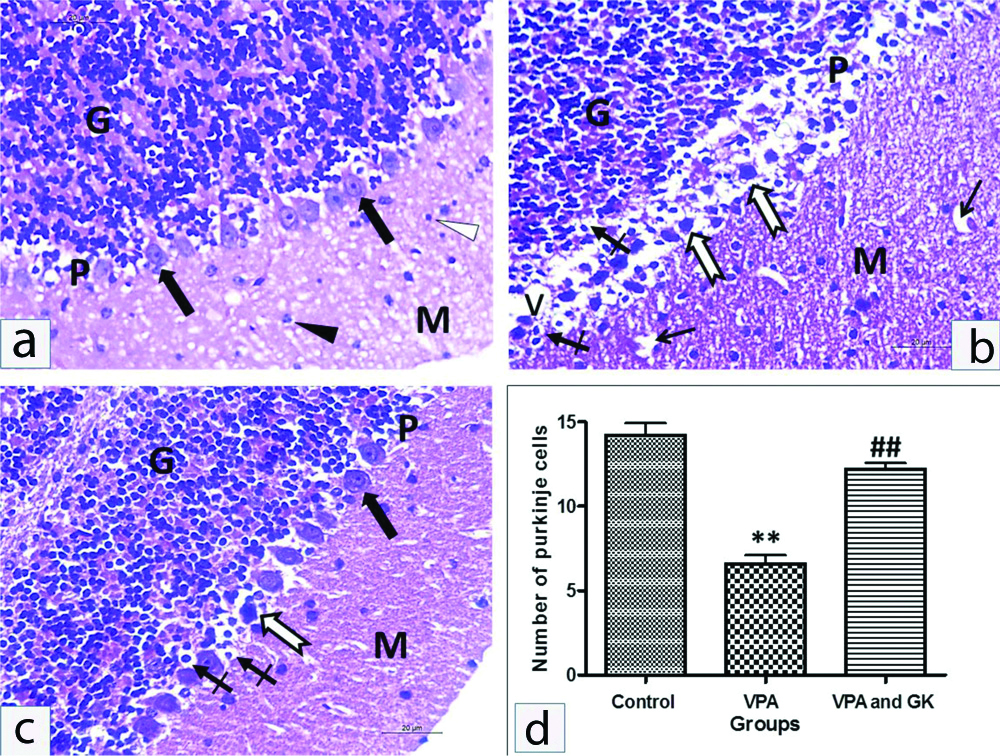
The cerebellum of control mice consisted of; molecular layer had mainly fibers with a few glial cells. The PCs were arranged in a single row contained well defined rounded vesicular nuclei and prominent nucleoli. Their cytoplasm was pale acidophilic and contained basophilic granules. The granular layer was stuffed with well-defined clumps of rounded cells with rounded deeply stained nuclei and scanty acidophilic cytoplasm. VPA-treated mice showed widespread neuronal affection, specifically of the PC layer. It revealed disturbed normal linear organisation with marked disarrangement. They appeared shrunken with pericellular unstained haloes. The cytoplasm of these cells showed eosinophilic homogenisation with irregular darkly stained nuclei. Some nuclei also showed pyknosis. The number of PCs was significantly decreased compared to the control group (6.6±1.14 vs 14.2±1.64, p<0.001). In parallel with morphological alterations seen in the Purkinje layer, the molecular layer displayed vacuolated areas. The granular cell layer showed pyknosis of their nuclei with pericellular unstained haloes. In VPA+GK group showed a few PCs affected in between the normal ones. The affected cells showed pyknosis. The number of PCs was up-regulated compared to the VPA group (12.2±0.84 vs 6.6±1.14, p<0.001). The granular cell layer exhibited apparently normal cerebellar islands but few cells showed pyknosis [Table/Fig-5].
Representative H&E staining of mice cerebellar cortex of different groups. The cerebellar cortex of the control group: (a) Formed of three layers; outer molecular layer (M), middle Purkinje cell layer (P) and inner granular cell layer (G). The outer molecular layer consisted of nerve fibers, small stellate cells (white arrowhead) and few scattered nuclei of basket cells (black arrowhead). The Purkinje cell layer consisted of one row of flask-shaped cells (thick black arrows) with large, rounded and vesicular nuclei in pale cytoplasm. The granular cell layer contained numerous compactly disposed granule nerve cells with darkly stained nuclei surrounded by very little cytoplasm. VPA treated group; (b), Purkinje cells appeared shrunken, disfigured with condensed chromatin (thick white arrows) and surrounded with vacuolated spaces (V). The nucleus of some of Purkinje cells and granulosa cells have been experienced pyknosis (crossed arrow). The granular layer (G) formed of densely packed cells. The molecular layer showed deeply stained pyknotic scattered basket cells and vacuolations (thin black arrows). Many Purkinje cells in the Ginkgo biloba protected group; (c) showed nearly normal appearance (thick black arrow). However, few of the Purkinje cells appeared shrunken, disfigured with condensed chromatin (thick white arrow), few Purkinje cells and granulosa cells showed pyknosis (crossed arrow). The number of Purkinje cells; (d) were dramatically decreased in VPA treated group; **p<0.001, compared with control. This decrease was significantly increased in the Ginkgo biloba extract protected group; ##p<0.001, compared with VPA treated group. Scale bar 50 μM, ×400.
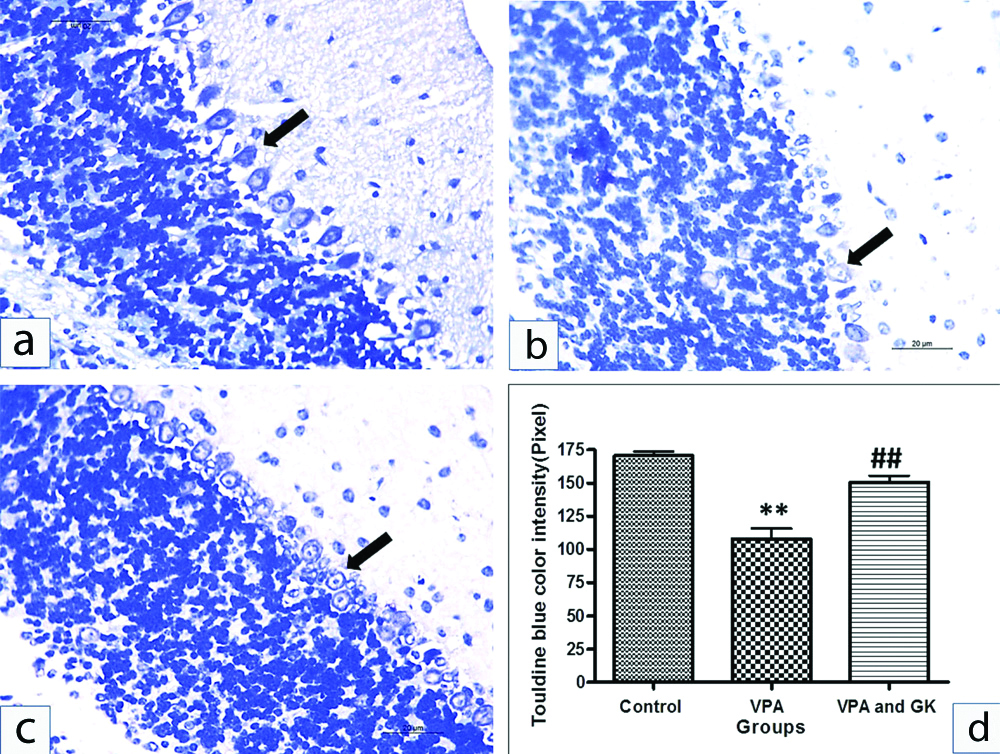
There was a significant decrease in colour intensity of toluidine blue staining in the PCs (Nissl’s granules content) in VPA group compared to control (107.8±18.14 vs 170.6±6.88, p<0.001). VPA+GK significantly up-regulated the toluidine blue colour intensity compared to VPA group (150.6±11.06 vs 107.8±18.14, p<0.001) [Table/Fig-6].
Representative toluidine blue staining of mice cerebellar cortex of different groups: the Purkinje cells of the control group (a) showed dark blue staining, Purkinje cells of VPA; (b) revealed a significant decrease in the colour intensity as compared to the control group. Toluidine blue staining in VPA+GK; (c) was up-regulated compared with VPA treated group. Statistical analysis; (d).**p<0.001, compared with control group, ##p<0.001, compared with VPA treated group. Scale bar 50 μM, ×400.
VI-Immunohistochemical Results
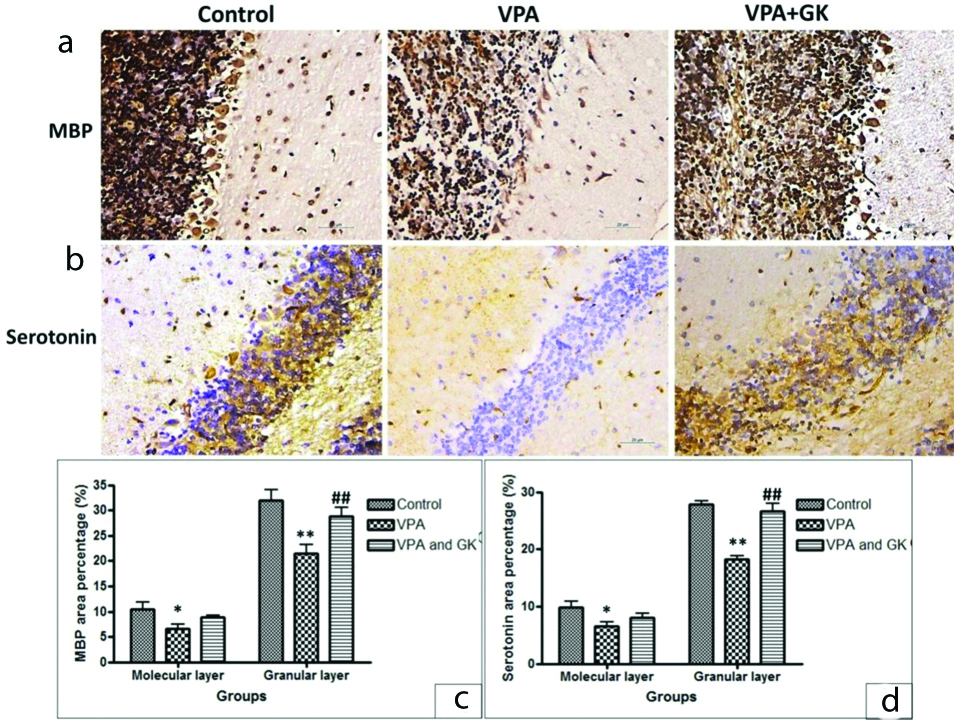
A significantly decreased expression of MBP; a marker of myelination was found in VPA group in both molecular and granular layers (6.6±2.07 vs 10.4±3.05 and 21.4±3.98 vs 32±4.53, p<0.05 and p<0.001, respectively). This expression was up-regulated in VPA+GK group compared to VPA group, especially in the granular layer (8.8±0.84 vs 6.6±2.07 and 28.8±3.96 vs 21.4±3.98, p>0.05 and p<0.001, respectively) [Table/Fig-7]. Serotonin expression was down-regulated in VPA group in both molecular and granular layers as compared to the control group (6.6±1.67 vs 9.8±2.59 and 18.8±1.03 vs 29.8±1.48 p<0.05 and p<0.001, respectively). This expression was up-regulated in VPA+GK group compared to VPA group, especially in the granular layer (8.0±1.71 vs 6.6±1.67 and 27±2.92 vs 18.8±1.03, p>0.05 and p<0.001, respectively) [Table/Fig-7].
(a,b) Representative MBP and serotonin staining of mice cerebellar cortex of different groups. There was down-regulation of MBP and Serotonin expression in VPA treated group, GinkgoBiloba Extract significantly up-regulated their expression. Statistical analysis (c,d), *p<0.05 and **p<0.001, compared with control group, ##p< 0.001, compared with VPA treated group. Scale bar 50 μM, ×400
Discussion
The quantitative measure of social interaction is the first defining feature of autism. Present study results demonstrated significant impairment of social interaction behaviour in VPA group when compared with the control group. This result was consistent with other reported studies [19]. All individuals with autism display repetitive behaviours [20]. Present study revealed a significant decrease in the percentage of alteration of T-maze test in VPA group when compared with the control group, indicating repetitive tendencies. This result was agreeable with other studies [21].
Concerning open field test, present study results demonstrated significant behavioural changes in VPA group as the number of crossing slots and times of center entries was significantly decreased when compared to the control group. These results were in agreement with other reported data [19]. However, the number of rearing was significantly higher when compared to the control group. This result was consistent with other studies [22]. On the other hand, VPA and GK group showed significant improvement in all disturbed behaviours except the number of crossed slots in the open field. These results were in agreement with the previously reported results that ginkgo biloba extract is a useful anxiolytic drug in the treatment of psychiatric diseases [23]. To identify the underlying mechanism of the ameliorative effect of GK; inflammatory and oxidative stress markers were measured.
In the current study; the inflammatory markers (IL-6 and IL-17) were significantly increased, however, the TGF-β1 level was significantly decreased in VPA group when compared to the control group. Similar results were reported previously [24]. Elevated IL-6 is responsible for demyelination in the cerebellum and also for autistic-like behaviours detected in present study. Elevation of IL-6 modifies both excitatory and inhibitory synaptic formations resulting in an abnormal change in the length, shape and distributing pattern of dendritic spines. Also, it disturbs the synaptic transmission [25].
IL-17 cytokines control the inflammatory responses by triggering the secretion of proinflammatory cytokines (IL-1β, TNFα, and MMP9) which causes neurotoxicity and neuronal death by an apoptotic mechanism and chemokines [26] which was evidenced in index histopathological results. Lower TGF-β1 levels were associated with worse behavioural symptoms in autism [27] which go hand in hand with present study results. On the other hand, the administration of Ginkgo biloba led to a significant reduction in the inflammatory state. The decreased levels of IL-6 in response to Ginkgo biloba may involve the regulation of NF-κB [28]. Ginkgo biloba alleviates VPA- induced autistic manifestations by reducing IL-17 production, and increasing levels of TGF-β1 that can down regulate inflammation [29].
In the current study, the brain MDA level was significantly increased while GSH was significantly decreased in VPA group when compared with the control group. These results were in agreement with other reported studies [30]. GSH is involved in neuroprotection in autism by improving the anti-oxidative stress system. MDA is used as a marker of lipid peroxidation, which is a chain reaction between polyunsaturated fatty acids and Reactive Oxygen Species (ROS), which are highly toxic to the cell [31].
Ginkgo biloba ameliorated the oxidative damage caused by VPA and this was in accordance with Singh SK et al., [23]. The neuroprotective effects of Ginkgo biloba may be attributed to its powerful anti-oxidant activity in lowering MDA in the brain. Also, the flavonoid fraction of Ginkgo increases the resistance of neurons to oxidative stress-induced injury by the induction of the anti-oxidant enzymes and reduces monoamine oxidase B activity, which is a source of ROS [32]. Furthermore, Ginkgo biloba improves neuronal membrane plasticity, neurogenesis, synaptogenesis, learning ability and cognitive function [33]. This is in accordance with present results which revealed improvement in memory, behaviour, cognitive aspects and neuronal cell death.
Moreover, Ginkgo biloba can decrease the expression of the gene encoding for Inducible Nitric-Oxide Synthase (iNOS); involved in neuroinflammation, thought Inhibition of NFκB [34]. Therefore, Ginkgo biloba may play a dual role as an anti-oxidant and an anti-inflammatory agent. In accordance with the behavioural and biochemical changes detected, index histopathological data revealed a significant PC degeneration and cell loss in VPA group. Similar results were reported previously [35]. However, mice treated with VPA and Ginkgo biloba showed significant increase in the number of PCs. This finding may be explained by the anti-oxidant capacity of Ginkgo biloba extract [36].
The present study revealed a significant decrease in the Nissl granule content in VPA group. This was in agreement with Kataoka S et al., [19]. Present study results also showed that the expression of MBP was significantly down-regulated in VPA group. Several lines of evidence support anti-MBP antibodies plays important role in the pathogenesis of autism [37]. Besides, present study results revealed that Ginkgo biloba significantly increased the Nissl granule content of the PCs and dramatically up-regulated the MBP expression. These results were confirmed by Ao Q et al., [38].
There was a down-regulation of serotonin expression in the cerebellum of VPA group. This could be explained by high serotonin (5HT) level in the whole blood at the early stages of brain development; entering the brain of a developing foetus and causing loss of 5-HT terminals through a negative feedback mechanism that causes a low intra-cerebral 5-HT concentration in ASD individuals. This loss of 5-HT innervations persists throughout subsequent development. Thus, the symptoms of autism appear [39]. On the other hand, Ginkgo biloba was able to up-regulate the serotonin expression in the cerebellum. This result was consistent with other studies that reported the ability of Ginkgo biloba to increase the serotonin level in several parts of the brain [40].
Limitation(s)
Lack of separation and identification of the active ingredients, responsible for the immune modulating and anti-oxidative stress effects of Ginkgo biloba extract by using high performance liquid chromatography technique was one of the limitations in this study.
Conclusion(s)
It can be concluded that Ginkgo biloba extract was capable of attenuating VPA induced changes on memory, behavioural and cognitive aspects and neuronal cell death via reducing inflammatory state, restoring the oxidative balance, improving nerve myelination and normalising cerebellum serotonin.