Bortezomib Induced Paralytic Ileus: Are we Aware?
Shailendra Prasad Verma1, Avaneesh Shukla2, Punita Pavecha3, Durga Prasad Verma4, Rashmi Kushwaha5
1 Associate Professor and Head, Department of Clinical Hematology, King George’s Medical University, Lucknow, Uttar Pradesh, India.
2 Senior Resident, Department of Clinical Hematology, King George’s Medical University, Lucknow, Uttar Pradesh, India.
3 Senior Resident, Department of Clinical Hematology, King George’s Medical University, Lucknow, Uttar Pradesh, India.
4 Senior Resident, Department of Clinical Hematology, King George’s Medical University, Lucknow, Uttar Pradesh, India.
5 Professor, Department of Pathology, King George’s Medical University, Lucknow, Uttar Pradesh, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Shailendra Prasad Verma, 1/167, Vikas Nagar, Lucknow, Uttar Pradesh, India.
E-mail: drspkgmu@rediffmail.com
Bortezomib is one of the most commonly used drugs in the treatment of multiple myeloma. It has many well-known side effects like peripheral neuropathy, thrombocytopenia and diarrhoea. Paralytic ileus has been rarely reported in patients of multiple myeloma receiving bortezomib and one should be aware about this entity. Stool frequency should be carefully monitored and the drug should be stopped timely to prevent complications of paralytic ileus. The patient was admitted for management of multiple myeloma and supportive care. He was started on the VTD (Bortezomib/thalidomide/dexamethasone) protocol. He developed abdominal distension and absolute constipation soon after the 2nd weekly dose of bortezomib. Abdominal X-ray revealed grossly dilated large bowel loops. Although he was given lactulose, glycerine suppository enema, the problem of abdominal distension and constipation persisted. His gastrointestinal symptoms improved and he was able to pass stools after bortezomib was removed from the protocol. One should be aware of this rare side effect of bortezomib. Bortezomib dose should be modified or it should be stopped timely to prevent complications.
Constipation, Multiple myeloma, Supportive care
Case Report
A 68-year-old male presented to theOut Patient Department of the Department of Clinical Hematology with complaints of backache for the past 25 days and altered consciousness for 2 days. He did not have any comorbidities like diabetes mellitus, hypertension, hypothyroidism, ischemic heart disease or chronic obstructive pulmonary disease. At presentation, he had mild pallor but no lymphadenopathy or organomegaly. There was no neck rigidity and his planters were bilaterally nonelicitable. His laboratory workup during admission is given in [Table/Fig-1] below. Based on bone marrow findings of 40% plasma cells [Table/Fig-2], serum protein electrophoresis and immunofixation studies, he was diagnosed as a case of multiple myeloma (ISS-Stage III, light chain disease). At the time of admission, he had decreased Serum potassium levels (2.05 mmol/L). His baseline renal function was also deranged (serum creatinine 1.78 mg/dL). He was given supportive care, renal safe antibiotics, and intravenous fluid along with IV potassium. His electrolyte imbalance improved by Day 2 and renal function was normal within a week. Patient became fully conscious oriented on Day 5 of admission.
Showing baseline Investigations of the patient during the hospital stay.
| Investigations | Results |
|---|
| Haemogram | Haemoglobin 9.6 gm/dL, TLC:9700, DLC:N68;L25;E04;M03;B00, Platelet count:1.76 lac/dL, CBC- RBC’s Showed normocytic normochromic anaemia with rouleaux formation. |
| LFT | S.Bilirubin:Total/Direct/Indirect:0.38 mg/0.19 mg/0.19 mg/dL, SGOT/SGPT:51U/31.2 U, Serum Alkaline phosphate:492.2, S protein/S albumin:5.05 gm/dL/3.08 gm/dL, S urea:39.8 mg/dL, S creatinine:1.59 mg/dL |
| Serum protein electrophoresis | Total IgA:1.410 g/dL, Total IgG:14.80 g/dL, Total IgM:0.72 g/dL, Serum light chains1) Kappa free light chain:38.50 mg/L; 2) Lambda free light chain:4010 mg/L, Kappa/Lambda ratio:0.010, Beta 2 microglobulin: 9784 ng/mL |
| HRCT thorax | Mild bilateral pleural effusion with subsegmental collapse & consolidation of the basal region of both lungs. Multiple lytic foci in all visualised bones seen. |
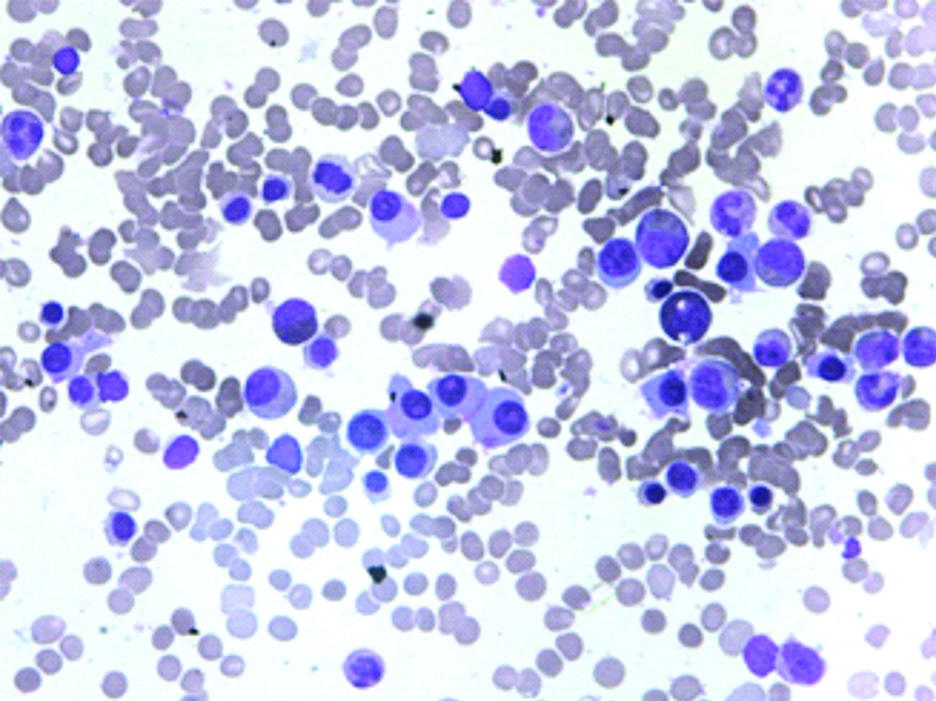
| Bone marrow [Table/Fig-2] | Bone marrow shows 40% plasma cells with occasional plasma blast |
TLC: Total leukocyte count; DLC: Differential leukocyte count; N: Neutrophils; L: Lymphocytes; E: Eosinophils; M: Monocytes; B: Basophils; CBC: Complete blood count; SGPT: Serum glutamic-pyruvic transaminase; SGOT: Serum glutamic-oxaloacetic transaminase; LFT: Liver function test; HRCT: High resolution computed tomography
Bone marrow aspirate (100x) showing increase in plasma cells (upto 40%) with occasional plasmablasts.
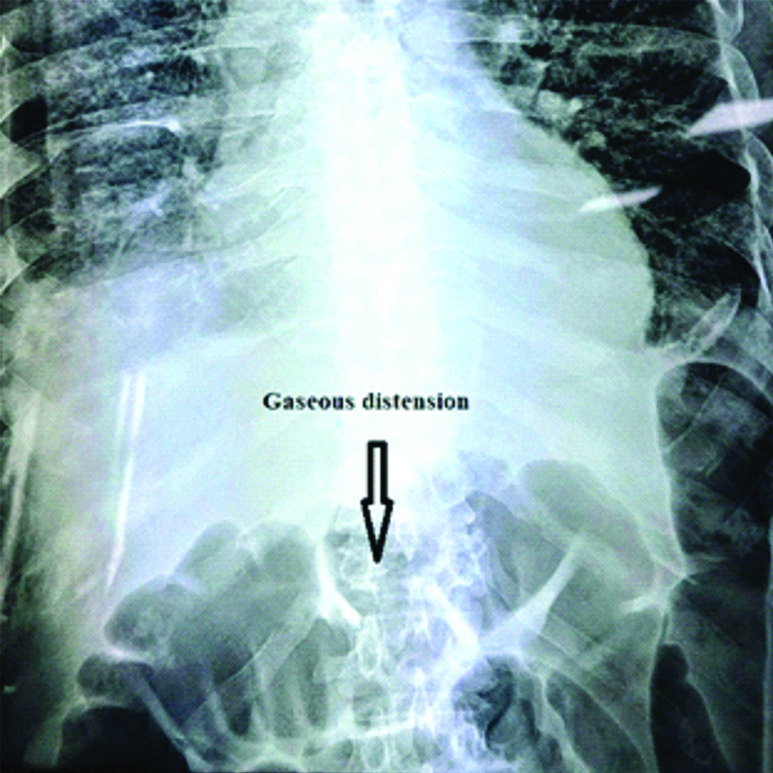
Patient was started on VTD (Bortezomib, thalidomide, dexamethasone) protocol on Day 5 of admission with weekly subcutaneous bortezomib schedule and low dose aspirin. He was given first dose of bortezomib on 18th March 2020 and later doses given on weekly basis. Post 2nd dose, Day 5 he complaint of abdominal distension with reduced frequency of defecation followed by absolute constipation over a period of 2 days. All oral medications were stopped. During this period he was afebrile and his electrolytes, serum amylase, serum lipase were normal. He was not taking any opiates, anticholinergic drugs or calcium channel blockers. His-thoraco-abdominal X-ray (PA view) showed grossly distended large bowel loops along with lower lung zone infiltrates and multiple osteolytic lesions in ribs [Table/Fig-3]. He was given intravenous Proton Pump Inhibitors; enema and flatus tube placement to manage gastrointestinal side effects but his complaints persisted. Finally, bortezomib was stopped from 3rd dose onwards. His symptoms improved gradually over one week. He was started on lenalidomidea long with low dose dexamethasone on 08th April for further management of multiple myeloma. The patient developed hospital-acquired pneumonia and expired during the hospital stay after 2 weeks of initiation of lenalidomide.
Thoraco-abdominal X-Ray (PA view) showing grossly distended large bowel loops along with lower lung zone infiltrates and multiple osteolytic lesions in ribs.
Discussion
Multiple myeloma is a common haematological malignancy in elderly. Recently, many new drugs and very effective protocols have significantly prolonged the survival of myeloma patients. Bortezomib based triple drug regimens are the most commonly used in upfront setting of newly diagnosed multiple myeloma. It has many well-known side effects like peripheral neuropathy, thrombocytopenia and diarrhoea. Paralytic ileus has been rarely reported in patients of multiple myeloma receiving bortezomib [1,2].
Bortezomib acts through nuclear factor-KB (NF-KB) pathway and inhibits degradation of abnormal cellular protein through proteasome system resulting in cell death. Along with the most common adverse events mentioned above, bortezomib also less frequently the peripheral and autonomic nervous system. Despite these side effects, autonomic gastrointestinal involvement is quite less especially with weekly subcutaneous bortezomib schedules. Autonomic nerve injury can also manifest as gastrointestinal side effects which are typically mild [1].
The gastrointestinal adverse effects of bortezomib were evident in a metacentric study published in the year 2006. In pooled data from 228 patients treated with bortezomib (1.3 mg/m2) in phase II clinical studies, the most common gastrointestinal adverse effects were nausea (64%), diarrhea (51%), constipation (43%), and vomiting (35%). In this study, paralytic ileus was not reported indicating that it is an uncommon occurrence during the treatment of multiple myeloma [3].
In another retrospective study of 19 patients with multiple myeloma, it was found that 2 patients had to discontinue their treatment due to bortezomib-induced paralytic ileus and toxic megacolon. Stool softeners, laxatives, hydration, etc., may be used to treat bortezomib-induced paralytic ileus but in two cases it was relieved by discontinuation of bortezomib [1].
Only case reports existed before 2016, describing paralytic ileus as a very rare side effect of subcutaneous bortezomib. Skin nerve biopsy and immunofluorescence study has demonstrated objective evidence of autonomic nerve fibre involvement with bortezomib use. Autonomic nerve fibre injury probably plays a significant role in the occurrence of this gastrointestinal side effect. In a prospective case series using the Naranjo questionnaire, 2 out of 18 patients developed recurrent paralytic ileus [3].
A case report published in 2007 reported that bortezomib may lead to progressive constipation. This may lead to paralytic ileus rarely. Every other cause of paralytic ileus was ruled out and eventually, it was seen that bortezomib is the cause of paralytic ileus which improves after stopping bortezomib [4].
Most studies have reported the development of paralytic ileus after a few cycles of bortezomib therapy. The present case had developed complaint of constipation soon after starting bortezomib. Attempts were made to treat constipation by using stool softeners, hydration and enema. The complaints of constipation persisted despite all efforts. The patient got relieved after stopping bortezomib. A single study comprising of two cases reported the development of paralytic ileus 12 and 15 days after the beginning of bortezomib. It was also seen that these patients were taking azole antifungals along with bortezomib. Bortezomib (1.3 mg/m2) was given on twice a week schedule (Days 1, 4, 8, and 11) supplemented with oral solution of Iitraconazole or voriconazole on daily basis [5].
The above discussion and review of literature clearly provides an important message to the treating physicians, oncologists and haematologists that bortezomib induced paralytic ileus is an uncommon but important side effect of this drug.
Conclusion(s)
Paralytic ileus may be a rare side effect of bortezomib. Usually, it is difficult to reach the cause of paralytic ileus as the patient may present with a lot of comorbidities, electrolyte abnormalities and spinal cord compression. One should keep in mind the possibility of bortezomib associated paralytic ileus if the patient develops abdominal distention, pain and constipation following initiation of bortezomib. There is a need for better proteasome inhibitors which are devoid of such side effects of bortezomib.
TLC: Total leukocyte count; DLC: Differential leukocyte count; N: Neutrophils; L: Lymphocytes; E: Eosinophils; M: Monocytes; B: Basophils; CBC: Complete blood count; SGPT: Serum glutamic-pyruvic transaminase; SGOT: Serum glutamic-oxaloacetic transaminase; LFT: Liver function test; HRCT: High resolution computed tomography
Author Declaration:
Financial or Other Competing Interests: None
Was informed consent obtained from the subjects involved in the study? Yes
For any images presented appropriate consent has been obtained from the subjects. Yes
Plagiarism Checking Methods: [Jain H et al.]
Plagiarism X-checker: Jun 01, 2020
Manual Googling: Jun 24, 2020
iThenticate Software: Jul 28, 2020 (13%)
[1]. Mele G, Coppi MR, Melpignano A, Quarta G, Paralytic ileus following “subcutaneous bortezomib” therapy: Focus on the clinical emergency-report of two casesClin Exp Med 2016 16(1):99-101.10.1007/s10238-015-0337-625600700 [Google Scholar] [CrossRef] [PubMed]
[2]. Keating M, Dasanu CA, Strategy to reduce bortezomib-induced paralytic ileus in patients with myeloma and impaired renal functionCase Reports 2016 2016:bcr20162170000.1136/bcr-2016-21700027899388 [Google Scholar] [CrossRef] [PubMed]
[3]. San Miguel J, Blade J, Boccadoro M, Cavenagh J, Glasmacher A, Jagannath S, A practical update on the use of bortezomib in the management of multiple myelomaThe Oncologist 2006 11(1):51-61.10.1634/theoncologist.11-1-5116401713 [Google Scholar] [CrossRef] [PubMed]
[4]. Perfetti V, Palladini G, Brunetti L, Sgarella A, Brugnatteli S, Gobbi PG, Bortezomib-induced paralytic ileus is a potential gastrointestinal side effect of this first-in-class anticancer proteasome inhibitorEur J Gastroenterol Hepatol 2007 19(7):599-601.10.1097/MEG.0b013e32811ebffe17556909 [Google Scholar] [CrossRef] [PubMed]
[5]. Koda Y, Mori T, Shimizu T, Kato J, Yamane A, Sadahira K, Early-onset of paralytic ileus caused by simultaneous administration of bortezomib and azole antifungals in multiple myeloma patientsThe Japanese Journal of Clinical Hematology 2012 53(8):760-64. [Google Scholar]