Since the first cases of The Coronavirus disease 2019 (COVID-19) emerged in December 2019 in Wuhan city, China [1], the pathogen named severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [2], previously referred to as 2019-nCoV [3], rapidly spread throughout the world. The World Health Organisation characterised Covid-19 as a pandemic at the beginning of March 2020 [4]. As of May 25, 2020, a total of 5,404,512 laboratory-confirmed cases had been documented globally [5].
The SARS-CoV-2 transmission routes include contact transmission and direct transmission [6,7] from symptomatic or asymptomatic patients [8,9]. There is suggestive data (a preprint, non-peer reviewed publication) that viral aerosol particles are produced by individuals that have the COVID-19, even in the absence of cough [10]. In addition, aerosol and fomite transmission of SARS-CoV-2 is plausible, since the virus can remain viable and infectious in aerosols for hours and on surfaces for days, depending on the inoculum shed [11]. Dental professionals are at high risk for healthcare-associated infection. They are potential carriers of a disease, and this is because of the aerosol generation and proximity of the provider to the patient’s oropharyngeal region [12].
Rational/appropriate Personal Protective Equipment (PPE) use, especially for respiratory protection, represents an important barrier to be adopted by healthcare workers, especially by dental personnel. In this sense, face/surgical masks or respirators (N95 or FFP2) should be used according to setting, personnel and activity type [13-15]. However, alternative solutions such as cloth (cotton or gauze) masks arise in view of challenges to the reserve medical supply system for public health emergencies. These personal barriers were used by healthcare workers during the SARS outbreak in China [16,17], and they are a popular choice, particularly in the developing world because they are inexpensive, locally available, and washable [18].
Considering the current Covid-19 pandemic scenario, what respiratory protection should be used in dental care settings? Surgical masks and FFP2 respirators have been reported as possible secure choices [19,20]. However, there is little evidence regarding the morphological characteristics of facial masks/respirators and their relationship with the possibility of interacting with droplets in the face of pandemic situations. The aim of this study was to evaluate interfibrillar gaps of cloth mask, surgical mask and FFP2 respirator through SEM in order to perform a graphic simulation of SARS-CoV-2 respiratory droplets in the gaps among mask fibres.
Materials and Methods
This descriptive and exploratory laboratorial study was conducted at the Technical Textiles and Protection Products Laboratory of the Technological Research Institute (IPT) in the state of São Paulo, Brazil, in December 2019.
The study sample consisted of three respiratory protections: a cloth mask (one layer made of cotton); a surgical mask (Medix, Brazil) and a FFP2 respirator (Descarpack, Brazil). The central portion of each mask was initially cut with a scalpel blade so as to make a 1 cm2 fragment to prepare the specimen for analysis under SEM [Table/Fig-1]. The textile layers of each mask and respirator were fixed to a metal stub with double-faced carbon tape and gold-sputter coated using a vacuum metalising appliance (CPD 030, Baltec, Blazers, Liechtenstein), with a pressure of 0.01 mbar, 40 mA current, 60 mm working distance, coverage time of 60 seconds and average deposition thickness of 15 to 16 nm.
Identification of the central area (1 cm2) for cutting a fragment for analysis in Scanning Electron Microscopy (SEM). a) FFP2 respirator; b) Surgical Mask; c) cloth mask.
Once the preparation stage was completed, the specimens was taken to a SEM (model QUANTA 400 FEG of the FEI brand, with acceleration voltage of 15 kV, working distance of 12 mm and High Vacuum operating mode), and the central portions were recorded of each specimen at magnifications of 100, 500 and 1000x. The distance among threads was determined by EDS.
The photomicrographs were treated in an image editor (Adobe Illustrator/Version 2020 24.0.1) to superimpose the textile layers of the surgical mask (3 textile layers) and the FFP2 respirator (6 textile layers) to identify the remaining empty spaces. The graphic simulation of respiratory droplets in the interfibrillar gaps was performed for all tested specimens. Considering droplets are in the range of 0.5 to 12 μm [21-23], this study performed graphical analysis of droplets with the minimum (0.5 μm), intermediate (5.75 μm) and maximum (12 μm) sizes. Graphic simulation considered the existing scales in the images. Illustrator tools were used to design the droplets (Pen Tool), to resize droplets to micrometer scale (Scale), and to calculate the image area (Get Shape Area script for Illustrator).
Results
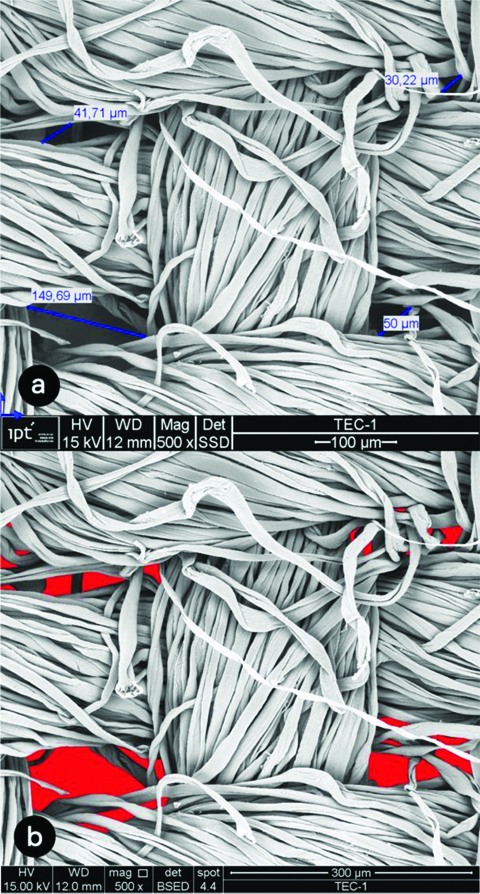
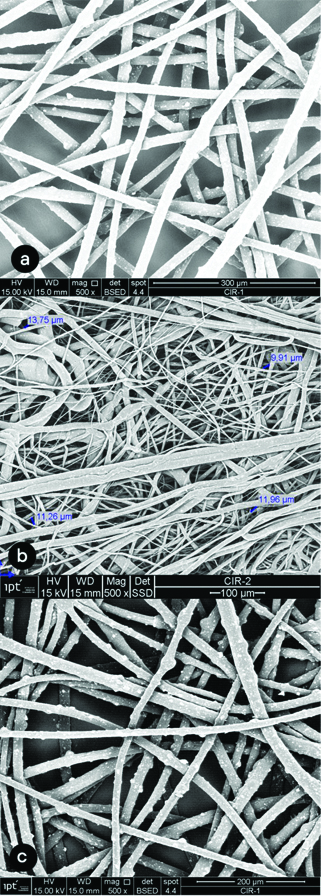
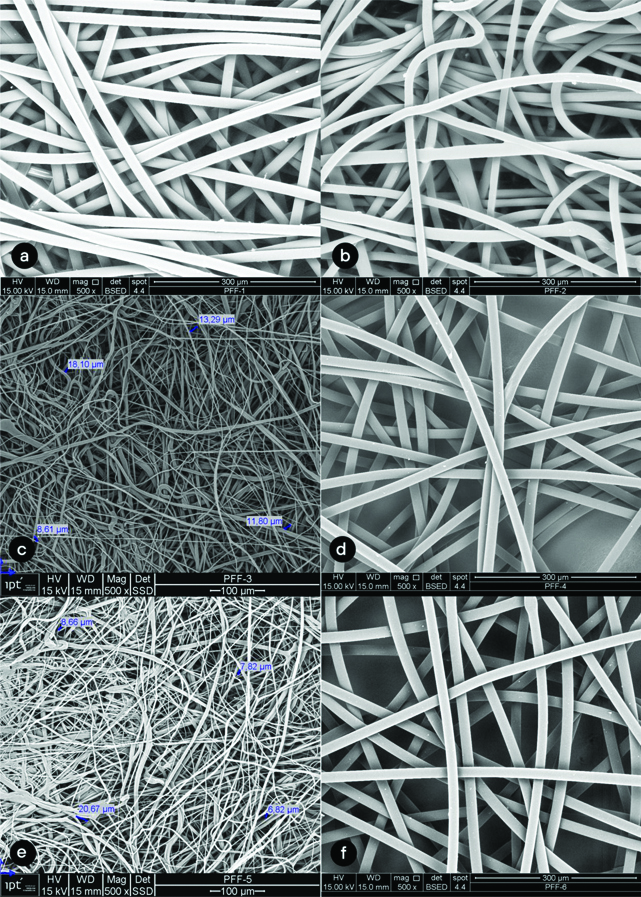
The image analysis of the cloth mask in close approximation (500x) showed the gaps among the cotton threads range from 30.22 μm to 149.69 μm [Table/Fig-2a,b]. The average size of gap was 67.91 μm. [Table/Fig-3] shows images of the surgical mask layers. The second layer presented closer interfibrillar spaces, whose distances ranged from 9.91 μm to 13.95 μm, with an average of 11.72 μm. The images of the six layers of the FFP2 respirator can be seen in [Table/Fig-4]. Layers 3 and 5 showed smaller interfibrillar spaces (6.82 μm-20.67 μm), with an average spacing between the fibrils of 11.97 μm.
(500 X) Photomicrographs of cloth mask. a) visualisation of distance among threads; b) gap spaces (red mark).
(500 X) Photomicrographs of surgical mask layers. a) layer 1; b) layer 2; c) layer 3.
(500 X) Photomicrographs of FFP2 respirator layers. a) layer 1; b) layer 2; c) layer 3; d) layer 4; e) layer 5; f) layer 6.
Interfibrillar empty spaces of surgical mask and FPP2 respirator are highlighted in [Table/Fig-5]. Remaining empty spaces were identified in surgical mask [Table/Fig-5a]. The superimposition of the textile layers of the FFP2 respirator did not exhibited any gap [Table/Fig-5b]. [Table/Fig-6] shows graphical simulation of the presence of minimum, intermediate and maximum size droplets in relation to the space of specimen fibrils of cloth mask, surgical mask and FFP2 respirator.
(500x) Graphic superimposition of surgical mask layers (a) and FFP2 layers (b). Empty spaces were identified in surgical mask, marked in red.

(1000 X) Graphic simulation of the presence of minimum (A), intermediate (B) and maximum (C) size droplets in relation to the space of specimen fibrils of cloth mask (a), surgical mask (b) and FFP2 respirator (c)
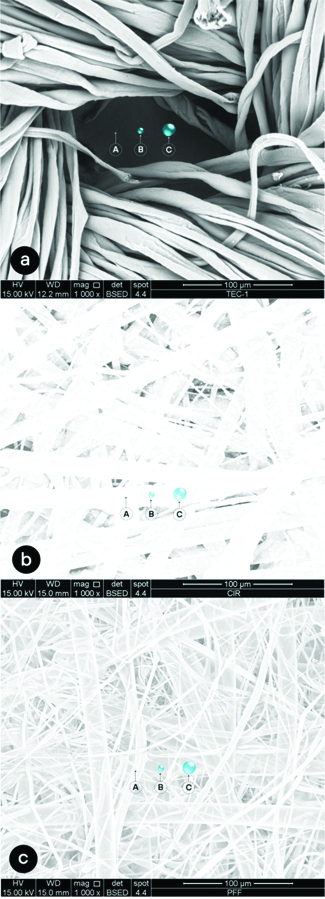
The area of weft gaps in the image with 500X magnification of cloth mask was 10,090.9 μm2, which represents 13.17% of the total area of the photomicrograph (76,592.07 μm2). The superposition of droplets in the gap areas indicated an overlap possibility of 36.327 minimum droplets, 278 intermediate droplets and 63 maximum droplets. In the surgical mask, on the other hand, the area of the spaces corresponded to 1,097.4 μm2, which represents 1.43% of the total area of the photomicrograph. In these spaces, 4389 minimal droplets, 33 medium droplets and no large droplets could be interposed. The transposition of these results to the scale of the total cloth mask area (189 cm2) would promote interposition of 8.9×109 minimum droplets, 6.8×107 intermediate droplets and 1.5×107 maximum droplets. In the spaces of surgical mask, 4389 minimal droplets, 33 medium droplets and no large droplets could be interposed. Results that would possibly represent the passage of 10,8×108 minimal droplets and 8,1×106 intermediate droplets. [Table/Fig-7] shows a graphical interposition simulation of intermediate droplets in the gaps in cloth and surgical mask.
(500 X) Graphic simulation of the presence of intermediate droplets (blue circle) on gaps. a) cloth mask. b) surgical mask, droplets marked in red.

Discussion
Dental care workers occupy the top positions among the professions most at risk for health because they have high rates of exposure to diseases and infections [24]. These professionals are exposed to direct contact with the oral mucosa, being one of the main routes of disease transmission. In this context, rotating instruments disperse large amounts of aerosols with microorganisms in the environment, making them contaminated [12], which represents a scenario of great concern in the COVID-19 pandemic.
Considering that SARS-Cov-2 has an approximate size of 0.05 to 0.2 μm [25], the first impression would be that there is unsatisfactory protection for the dental health care worker, since the discrepancy between the measures would probably allow the virus to pass through the fabric fibres [Table/Fig-2,3 and 4]. However, SARS-Cov-2 transmission is associated with respiratory droplets which vary from 0.5 to 12 μm. Thus, even the largest droplets would possibly be able to pass through the pores of the cloth masks. For the surgical mask and the FFP2 respirator, on the other hand, physical containment of droplets by the fibres of the protective equipment would occur due to the interposition of the different layers.
The number of layers is a factor regarding the filtration capacity of a cloth mask [16]. From this perspective, masks with several layers would also be more advantageous because they would promote greater electrostatic attraction, which predominately remove low mass particles attracted and bonded to the fibres [26]. Naturally, produced droplets from humans (i.e., droplets produced by breathing, talking, sneezing, coughing) include various cell types, physiological electrolytes contained in mucous and saliva, as well as various potential infectious agents [21]. Half of the droplets in a typical human cough may be small (<10 μm) [27]. In this sense, the FFP2 respirator would be the protection of choice to block droplets containing SARS-CoV-2 due to 6-layes structure. This investigation only analysed a one layer cloth mask. The addition of other layers could represent a greater structural barrier to the flow of particles. However, some studies have shown little protection capacity, even in cloth masks with more than one layer [17,18].
Our ultra-structural results complement the studies that evaluated filtration efficiency, which concluded that respirators (FFP2 or N95) promotes a superior filtration capacity, followed by surgical masks [18,28]. One study found that the filtration performance of facepiece respirators in a 2.5 μm particle flow was greater than 95%. A statistically similar value was obtained for the surgical mask, while the filtration of the cloth mask was around 70% [18]. However, this result did not consider the possibility of lateral air infiltration due to the lack of sealing. When this factor is taken into account, FFP2 masks provided adults with about 50 times as much protection as homemade masks, and 25 times as much protection as surgical masks [28]. Faced with the emergency of a virulent disease such as COVID-19, it would be logical to use a respirator (N95/FFP2) in dentistry as it offers resistance to fluid penetration and forms a seal around the mouth and nose in contrast to surgical masks that provide barrier protection only against droplets including large respiratory particles [29].
Although the area of the interfibrillar spaces indicates a worrying number of intermediate droplets in surgical mask (8,1×106), the existing gaps would allow the passage of a few intermediate droplets [Table/Fig-7]. In this case, the attractiveness of electrostatic forces would allow satisfactory filtration. For minimal droplets, responsible for the airborne transmission of microorganisms, the surgical mask would allow the passage of many particles, even under the influence of electrostatic forces. These results corroborate the indication that this PPE is not recommended for procedures with aerosol formation [13,14,19,29] which are recurrent situations in dental clinical practice.
The results showed that the transposition of over 680 million droplets (5.75 μm) would be possible through the investigated cloth mask. Although the concentration of SARS-CoV-2 in respiratory droplets is still unknown, the graphical interposition of droplets through cloth mask gaps [Table/Fig-7] suggests greater exposure to the risk of viral transmission. Cloth masks would provide the users little protection from microorganisms from other persons who are infected with respiratory diseases [23,22]. A randomised clinical trial of cloth masks concluded that these barriers should not be recommended for healthcare workers, since a higher risk for viral infection has been observed in individuals who used cloth masks in providing healthcare [17]. As large number of droplets and aerosols can be generated on dental procedures [30], probably the cloth mask would not provide an effective barrier against SARS-Cov-2 in dental settings.
First-line healthcare workers may be at risk, if no proper PPE is available [31], risk that can also be extended to oral health professionals by the constant presence of bioaerosols. Since the SARSCoV-2 outbreak, China has faced a shortage of medical supplies and has also received medical masks, protective clothing, goggles, and other materials donated by South Korea, Japan, Britain, France, and other countries [32]. Many countries are already facing this shortage in the current epidemiological scenario with the great impact of COVID-19 on a worldwide scale. This requires urgent measures aimed at producing and distributing personal protective supplies such as surgical masks and N95/FFP2 respirators. In addition, the findings are an alert and in some way support the World Health Organisation’s recommendation that cotton cloth masks are not considered appropriate for health care workers [33].
Limitation(s)
The descriptive and exploratory nature of this study places the micromorphological analysis of a single sample for each group as a limitation of the study. From this perspective, future studies are needed to perform comparative evaluation through inferential statistical analyses with a larger number of specimens.
Conclusion(s)
The results are not intended to verify the effectiveness of protective equipment or even to establish a recommendation for use by healthcare workers. The main objective of this investigation was to visually demonstrate what can happen at the microscopic level when using cloth mask, surgical mask and respirator FFP2 against the infectious particles of COVID-19. The graphic simulation of SARS-CoV-2 respiratory droplets demonstrated that the droplets (0.5-12 μm) would possibly be able to pass through the fabric pores of a cloth mask, which could represent a risk for viral infection. The SEM images showed cloth mask presented higher gaps among the fabric fibres than surgical mask and FFP2 respirator. The superimposition of the layers of the FFP2 respirator showed no spaces between the fibres, which would promote the blockage of the droplets in at least one of the layers.