Ceftriaxone is a broad-spectrum third generation Cephalosporin used to treat as well as prevent bacterial infections in both medical and surgical conditions [1]. The ease of administration as well as relative paucity of adverse effects makes it one of the most frequently administered antibiotics in inpatients [1]. The drug, however, may cause precipitation of ceftriaxone calcium salts in gallbladder as well as within the urinary tract which usually resolve on discontinuation of therapy. These precipitates, termed pseudoliths were first identified sonologically by Schaad UB et al., in 1986 [2] and result due to hepatic excretion of the agent and interaction with bile salts and calcium. The concentration of ceftriaxone within gallbladder and due to its high calcium binding affinity, precipitation of calcium-ceftriaxone salt occurs within gallbladder [3,4]. This is compounded by inhibition of phospholipid and cholesterol secretion induced by endogenous or exogenous bile acids [4]. Various other factors predispose patients on ceftriaxone therapy to pseudolithiasis, most important of them being biliary stasis resulting either due to fasting or other conditions [5]. Most often, these pseudoliths are asymptomatic and resolve on discontinuation of therapy with a mean duration of 15 days [6], but a number of reports exist with patients presenting with symptoms due to these benign stones and a few undergoing surgical procedures too [7-9]. The present study envisaged to answer following questions: (i) incidence of pseudolithiasis in hospital patients treated with parenteral Ceftriaxone; (ii) assess resolution of these pseudoliths; and (iii) incidence of patients presenting with symptoms attributable to pseudolithiasis.
Materials and Methods
A prospective observational cohort study was conducted in a tertiary care centre in August 2016 patients were enrolled for next one year, till July 2017 and followed for a period of one year (June 2018). Ethics committee approval was taken to conduct the study with waiver of informed consent as all studies performed were as per standard of care at the institution and no active interventions were envisaged as part of the study (IEC/MHM/2016/05 dt 31 Jul 2016).
To calculate sample size for a qualitative variable (in an incident cohort design) with 10% taken as the incidence of pseudolithiasis [2,5,6], an absolute error at 5% and type 1 error at 5%, a minimum sample size of 1245 was envisaged.
Inclusion criteria: For the study were all inpatients started on parenteral ceftriaxone at the institute during this period.
Exclusion criteria: Patients who underwent prior cholecystectomy, those already diagnosed with gallstone disease or abnormal gallbladder findings on day 1 sonogram, patients younger than 18 years of age, patients already on any oral or parenteral antibiotics prior to admission and initiation of ceftriaxone therapy at the centre and patients with hepatic dysfunction (elevated serum aminases or bilirubin). Also, patients who are not willing to participate in study and those not available for follow-up examinations (due to death/discharge/transfer to another institute or absence of radiologist to perform the sonographic examination) were also not included in the study.
A total of 1811 patients (1193 males and 618 females) were taken up for the first sonographic examination on day 1 of therapy during this period [Table/Fig-1]. Patients admitted during the evening and started up on ceftriaxone were taken up for sonographic examination the next morning.
Flowchart of patients enrolled in the study.
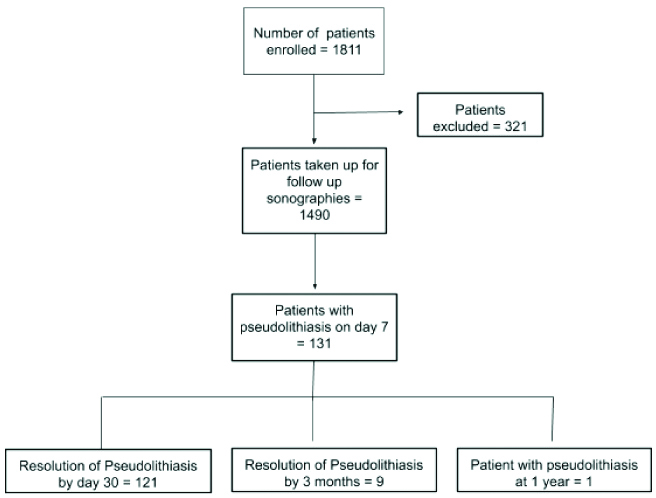
A total of 321 patients were excluded from the study due to reasons/exclusion criteria as mentioned in [Table/Fig-2].
Patients on ceftriaxone who were excluded from the study.
| Reason | Numbers |
|---|
| 1 | Cholecystectomy | 35 |
| 2 | Pre-existing gallstones | 47 |
| 3 | Patients already on antibiotics before admission at this institute | 106 |
| 4 | Laboratory parameters suggestive of hepatic dysfunction | 62 |
| 5 | Patients not available for follow-up examinations (due to death/discharge/transfer to other institutions/absence of first author to perform repeat investigation. | 71 |
The remaining 1490 patients (962 males and 528 females) were subjected to a second sonographic examination on day 7 of parenteral ceftriaxone therapy. Sonographic examinations were performed on Logiq C2 USG machine (GE healthcare, Wauwatosa, USA) or Mindray DP-5 (Shenzhen Mindray Bio-Medical Electronic Co., Ltd., Shenzhen, China). All scans were performed by the first author who had experience of doing sonographic examinations for over nine years at the time of initiation of study.
All patients who revealed pseudoliths on day 7 sonogram were followed-up with repeat sonographic examinations planned at one month, three months, six months and at one year from the initiation of therapy. Further sonographic studies were halted once resolution of pseudoliths was noted during follow-up. In addition, sonography was performed as and when patients presented with upper abdominal symptoms suggestive of biliary colic. The duration of therapy with parenteral ceftriaxone was also not taken into consideration while assessing for development of pseudolithiasis.
The incidence of Pseudolithiasis was calculated using standard statistical formula as under:
Number of cases developing pseudolithiasis on day 7 sonogram x 100 ÷ Number of patients included in the study.
The incidence of complications due to Pseudolithiasis was calculated using standard statistical formula as under:
Number of patients with pseudolithiasis with biliary symptoms x 100 ÷ Number of patients with pseudolithiasis.
Statistical Analysis
Descriptive statistics in terms of numbers and percentages were used.
Results
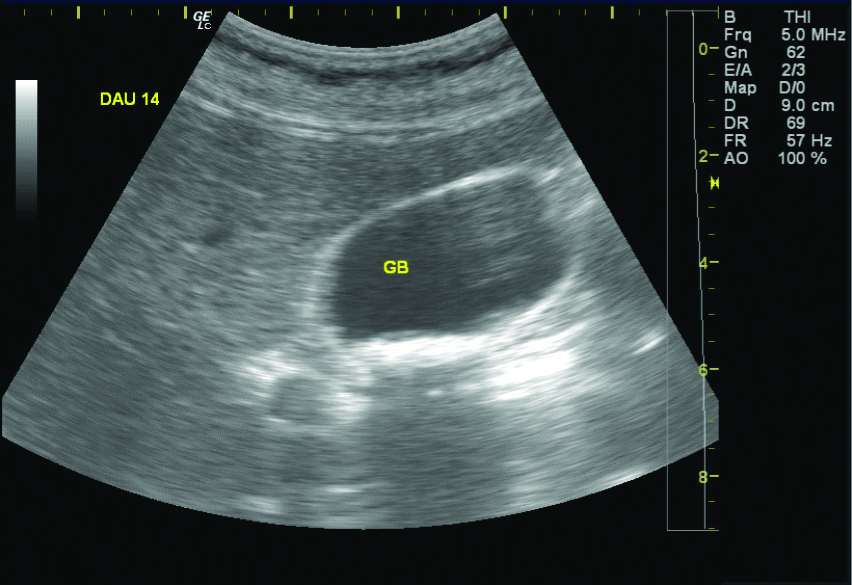
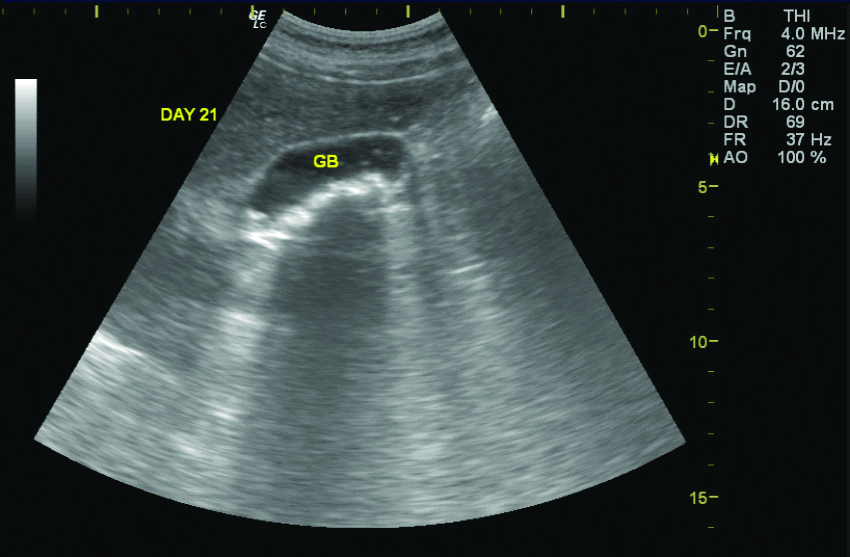
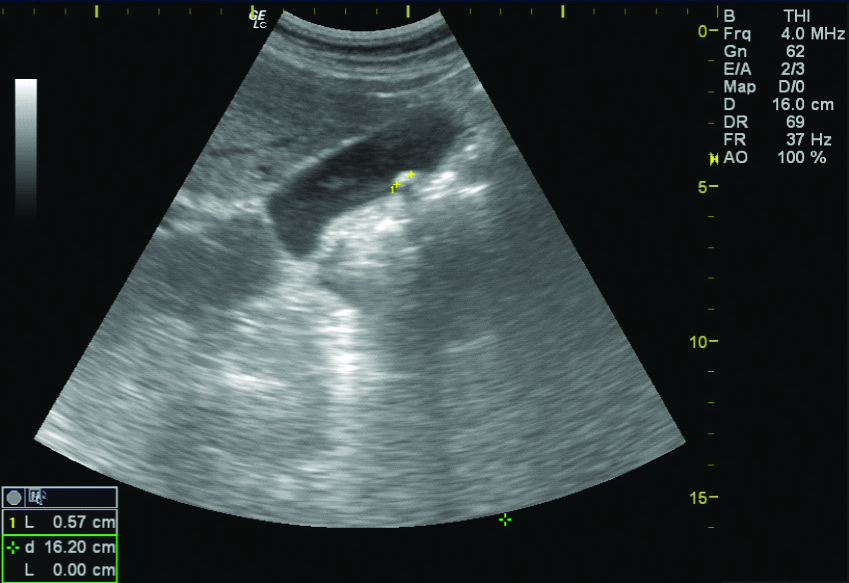
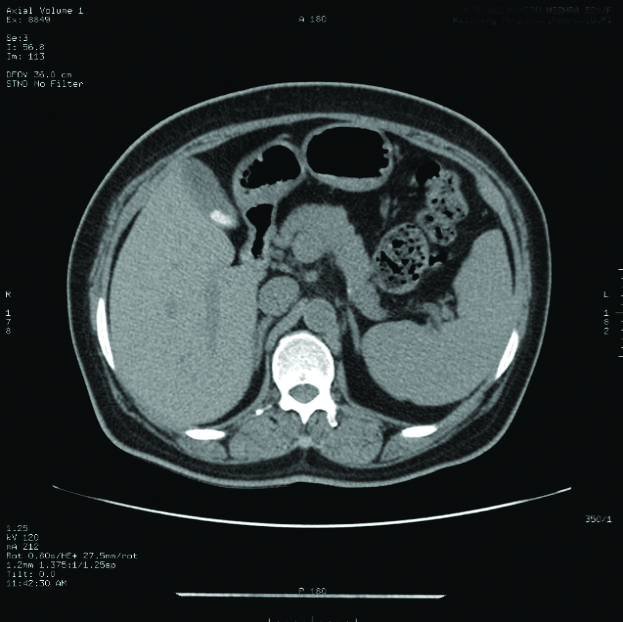
Of the 1490 patients included in the study, 131 (82 males and 49 females, 8.7%) patients revealed pseudolithiasis (on day 7 examination). These were most often seen as tiny echogenic mobile foci with distal acoustic shadowing [Table/Fig-3], variable sized calculi with faint distal shadowing and small echogenic foci with distal shadowing [Table/Fig-4], however other variations like large calculi with dense distal shadowing [Table/Fig-5], or echogenic foci within hypoechoic sludge were also seen. Occasionally, calculi were diagnosed on computed tomographic examinations performed before the end of 7 days as radiodense dependent contents seen in [Table/Fig-6] but the study protocol of assessing gallbladder on sonography after 7 days was still adhered to all cases.
Ceftriaxone calculi seen on sonography as tiny echogenic mobile foci casting distal acoustic shadowing.
GB: Gall bladder
Ceftriaxone calculi with echogenic mobile foci casting distal shadowing of distinct variable size (upto 10 mm).
GB: Gall bladder
A single echogenic mobile focus seen as casting dense distal shadowing.
Calculi picked up as hyperdense foci on CT images.
One hundred and twenty-one patients out of 131 (92%) patients with pseudolithiasis had resolution of pseudolithiasis when a repeat sonography was performed on day 30 from start of Inj ceftriaxone. Nine out of the remaining 10 patients revealed no pseudoliths on a second follow-up scan done after three months of initiating therapy [Table/Fig-1].
One patient (0.76%), a 27-year-old male, had persistent pseudolithiasis on all follow-up imaging performed till after one year of initiation of ceftriaxone therapy. While initial studies at one week and one month revealed multiple tiny echogenic mobile foci with distal shadowing, follow-up sonographic examinations showed only one small (5.7 mm sized) echogenic mobile focus with dense distal shadowing (same patient as in [Table/Fig-5]). Patient was asymptomatic throughout the period of study and the possibility of a pre-existing gallstone which was missed during the first sonographic examination cannot be ruled out.
Eleven patients out of 131 patients (8.3%) with pseudolithiasis and 0.73% of the total study population had new onset upper abdominal symptoms suggestive of biliary colic (epigastric or right hypochondrial pain without nausea or vomiting). This presentation was during ongoing ceftriaxone therapy in six patients (within two weeks of initiation of Inj ceftriaxone) while the rest (five patients) presented after discontinuation of therapy and before resolution of pseudolithiasis [Table/Fig-7]. Possibility of pre-existing illness resulting in these symptoms was excluded clinically. Sonographic examination at time of symptoms revealed pseudolithiasis but no evidence of cholecystitis or choledocholithiasis. All these patients improved with conservative management without need of a surgical procedure. None of these patients had persistence of pseudolithiasis when seen after three months of initiation of therapy.
Patients with upper abdominal symptoms due to pseudolithiasis.
| Complaints | Number of patients |
|---|
| Epigastric or right hypochondrial pain | 6 |
| Vomiting with right hypochondrial pain | 5 |
| Pancreatitis/cholecystitis | 0 |
| Choledocholithiasis | 0 |
Discussion
Formation of gallstones subsequent to parenteral Ceftriaxone was first reported by Schaad UB et al., in 1986 [2] in paediatric patients and due to spontaneous resolution after discontinuation of therapy, the term ‘reversible ceftriaxone biliary pseudolithiasis’ [10] was coined. The incidence of calculus formation is dependent on a number of known factors like dosage and duration of therapy, renal dysfunction, paediatric age group and biliary stasis (due to fasting or other conditions). An overall incidence of 5 to 25% amongst inpatients is considered usual and similar rates have been noted in smaller double-blind controlled studies [11]. Paediatric patients have a higher incidence of development of pseudoliths as observed by Papadopoulou F et al., with only a small percentage (4%) of them presenting with symptoms of biliary colic [12]. Though urolithiasis may also be seen in patients on therapy with Inj. ceftriaxone, the incidence of the same was not assessed during this study. The present study included a much larger number of adult patients, more than 10 times most other studies performed in the past. Also, the patients had no hepatic or renal dysfunction at the beginning of therapy as both these conditions predispose to further gallstone formation. An incidence of 8.79% was noted in present study with more than 90% of these patients showing complete resolution of pseudolithiasis within 30 days of initiation of therapy. The timeline of resolution corroborates with other studies done earlier [2,6,10]. Most patients who develop pseudolithiasis remain asymptomatic with serious complications like cholecystitis and pancreatitis occurring in a small minority. A number of case reports referring to such occasional complications are widely available in literature [13,14] and occur more frequently in patients with other co-morbidities like renal failure or patients on total parenteral nutrition. Only 11 patients (0.73%) patients presented with new onset upper abdominal symptoms which were clinically attributable to pseudolithiasis. All these patients had resolution of symptoms as well as pseudoliths with conservative therapy and no surgical intervention was required in any of these patients. None of the patients presented with cholecystitis or pancreatitis or obstructive jaundice. Though complications of pseudolithiasis are uncommon, further studies may be undertaken with a larger patient base so as to assess for nephrolithiasis, relationship with dosage of ceftriaxone and arrive at optimal management strategies.
Limitation(s)
The duration of ceftriaxone therapy, dosage of therapy and the time interval between cessation of therapy and resolution of pseudolithiasis was not included in this study. More frequent sonographic examinations after detection of pseudolithiasis would have given a greater insight into the natural course of these calculi, however, the same was not included in the present study. Only sonographic examinations were carried out to detect and follow calculi and no other modalities were considered in this study. Though all examinations were carried out by a trained radiologist and gallbladder was thoroughly evaluated during every study, sonography is not infallible in detection of gallstones/pseudolithiasis.
Conclusion(s)
Knowledge of this entity has certain important clinical and medico-legal implications for radiologists and treating physicians. Firstly, counselling patients about this condition as a complication of ceftriaxone therapy should be added. Detection of gallstones during or immediately after treatment with parenteral ceftriaxone can be due to the development of pseudolithiasis and not due to an erroneous sonographic report. As seen in present study, complications arising due to these pseudoliths are infrequent (less than 1% of patients) and active surgical intervention should be withheld till failure of conservative management or persistence of calculi and clinical symptoms after cessation of ceftriaxone therapy.